Tools for Managing Internal Parasites in Small Ruminants: Animal Selection
By Linda Coffey, NCAT Agriculture Specialist
Abstract
For long-term animal health, improving sheep and goat resistance or resilience to internal parasites is a very important strategy. Animal breeding can build a stronger, more resistant herd or flock if producers will identify and select the best animals for long-term health. This publication discusses methods and rationale for selecting sheep and goats with improved resistance or resilience to internal parasites. It also briefly describes other management tools helpful to producers and to the small ruminants raised in humid areas.

Animals can be selected for their resistance to parasites, resulting in a stronger flock. Photo: Linda Coffey, NCAT
Contents
Introduction
Animal Selection
Breeds
Measuring Resistance or Resilience
How to Use This Information
Encouragement
Summary
Internal Parasite Management Assessment
References
Further Resources
Introduction
Internal parasites are a major health problem for sheep and goats raised in humid areas, especially where land is limited. For years, anthelmintics have mitigated the effects of these parasites and enabled farmers and ranchers to maintain the productivity and health of their livestock. However, internal parasites have developed resistance to anthelmintics (dewormers). Today’s sheep or goat producer must use all available tools to help manage internal parasites.
Mature parasites breed inside the host and “lay eggs,” which pass through the host and are shed in the feces. After the eggs pass out of the host, they hatch into larvae. Warm, humid conditions encourage hatching of the eggs and development into infective larvae. The larvae need moisture, such as dew or rain, to break open the fecal pellet and move. They migrate out of the feces and travel up blades of grass. When an animal (sheep or goat) grazes, it may take in parasite larvae along with the grass blade. Parasite numbers increase over time when conditions are favorable (warm, wet). The larvae mature inside the host, and the cycle continues.
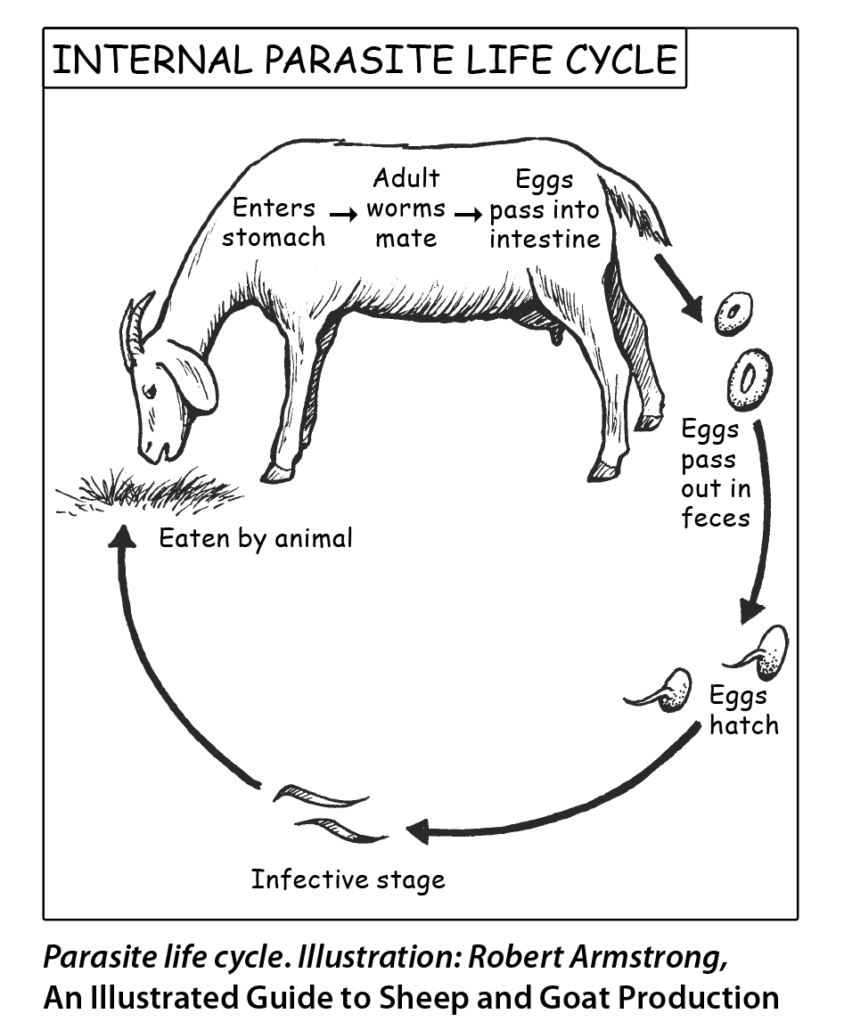
Adult internal parasites affect their host in various ways. They can damage the lining of the stomach or intestines, which can lead to weight loss and anemia, along with related symptoms such as weakness, bottle jaw, and anorexia (loss of appetite). Haemonchus contortus (barberpole worms) disrupt and damage the stomach lining and feed on blood, which can result in anemia. Other worms and coccidia cause intestinal lining damage, which can result in reduced absorption of nutrients and lead to scours (diarrhea) and weight loss or poor weight gain.
This publication is concerned with breeding resistance to gastrointestinal nematodes (roundworms). Coccidia are mentioned in passing, as they are important internal parasites in lambs and kids, and producers should be alert to the possibility of coccidia and get a good diagnosis so that effective treatments can be used.

Bottle jaw. Photo: J.M. Luginbuhl, NCSU

This goat shows signs of severe parasite infection.
Photo: J.M. Luginbuhl, NCSU

This goat appears healthy and in good condition. Photo: Linda Coffey, NCAT
When adult parasite numbers inside the host animal reach a level that causes obvious illness, producers have historically relied on anthelmintics (dewormers) to kill the parasites and allow the animal to heal and recover. However, as the animal grazes, it may be continually ingesting more parasite larvae, giving a new “crop” of parasites a home inside the animal. The presence of parasite larvae in the environment is often referred to as a “challenge,” and animals that can perform well in spite of the challenge are either resilient (tolerant) or resistant to internal parasites. Selecting animals that are resistant will lower the challenge on the farm over time. Selecting animals that are resilient may not impact the number of parasite larvae in the environment, but will result in better animal survival and production in the face of a challenge.
Is there a problem?
Signs of internal parasite infection commonly include some or all of the following. Note that some signs may be caused by other conditions as well).
- Poor growth or reduced milk production
- Loss in body condition (animal becomes thinner in spite of good nutrition)
- Rough hair coat or poor fleece
- Scouring (diarrhea: wet feces rather than pelleted; not seen with all parasites)
- Reduced vigor (animals appear lethargic and lag behind the flock or herd)
- Reduced appetite
- Anemia (seen in pale mucous membranes; caused by bloodsucking parasites, such as Haemonchus contortus)
- Bottle jaw
- Sudden death after a stress (e.g., an animal is chased on a hot, humid day)
Because internal parasites are so adaptable, difficult to control, and damaging to animal health, it is important that producers use every available tool to protect their livestock and keep internal parasite populations in check.
What can you do?
Strategies or tools that can be employed to fight internal parasite infection include:
- Good nutrition to support the immune system
- Selective deworming based on FAMACHA© or other criteria
- Pasture management
- Alternative control methods (e.g., botanicals, copper oxide wire particles)
- Selecting resistant animals
For more about these strategies, see the ATTRA publication Managing Internal Parasites in Sheep and Goats.
The remainder of this publication explores various aspects of selecting animals for internal parasite resistance.
Animal Selection
Resistance to internal parasites means that an animal exposed to internal parasites suppresses establishment of parasites inside the body, or suppresses fecundity (egg-laying) of the worms if they do establish. Shedding of parasite eggs will be minimal in a resistant animal, so a resistant animal will benefit the whole flock by reducing contamination of the farm.
Research has shown that internal parasites are not evenly distributed in a herd or flock. Often 80% of the internal parasites will be in 20% of the animals. This is referred to as the “80/20 rule.” If you can identify those animals harboring the most parasites and remove them from your herd, you can lower pasture contamination significantly. Also, because resistance is heritable, breeding those animals that are more resistant will result in a stronger herd over time. For example, one study found that Merino sheep that were selected for resistance had fecal egg counts (FEC) reduced by 69%. Also, the FEC in untreated selected sheep were lower than the FEC in strategically drenched unselected sheep; in other words, the effect of breeding was greater than the effect of strategic treatment (Eady et al., 2003). In an Australian study, Merino ewes selected for increased resistance to H. contortus had significantly lower egg counts at all times before and during the peri-parturient period, compared to ewes selected for susceptibility (Woolaston, 1992). Heritability in goats is thought to be lower and resistance is expressed later (at older ages), but selecting for resistance is still feasible and will result in lower pasture contamination over time (Vagenas et al., 2002).

Rams and bucks have a large impact on the parasite status of the farm. These Gulf Coast rams have never needed deworming. Photo: Linda Coffey, NCAT
Another possibility if there is room; mother and daughter grazing (goats).
Resistance is measured by taking fecal samples and doing quantitative fecal egg counts on animals that have not been dewormed in at least six weeks (preferably all animals treated or untreated similarly). Animals shedding fewer eggs are then identified and retained for breeding, while animals shedding the most eggs would be identified and then culled. Rams and bucks provide half of the genetic material for the lamb and kid crop, so choosing a more resistant sire would have a large impact on the parasite resistance and contamination level on the farm in years to come.
The problem with selecting for resistance is that sometimes production traits are negatively correlated with resistance (Bisset, 1996; Hoste and Chartier, 1993). Because stress impacts the immune system and makes an animal more susceptible to internal parasites, producers might observe that a doe that produces the most milk (causing a nutritional or metabolic stress) also has the most trouble with parasites. Also, lambs being raised as twins usually have a higher fecal egg count than those raised as singles (Wolf et al., 2008). Producers will have to balance the factors of observed internal parasite resistance and production traits and consider the whole farm system (Torres-Acosta and Hoste, 2008).
Breeds
Because of the variability mentioned earlier and the heritability, it is possible to make progress within a breed by focusing on resistance to internal parasites as a selection trait. Katahdin breeders are working on this now.
Additionally, there are some breeds that have been naturally selected for resistance to internal parasites. These breeds usually were developed in situations and climates that favored internal parasites. The animals were then selected by “survival of the fittest,” and they will be significantly more resistant on average than other breeds that were not raised under those conditions. A note of caution is in order: These resistant breeds will still have variability within their ranks, and each animal will need to be evaluated on its merits. On a pasture-based buck test in Oklahoma in 2008, the best buck and the worst buck for internal parasite resistance were the same breed.
It is possible to have parasite problems even though the breed is known to be resistant, and that resistance can be lost when the animals are no longer subjected to the same selection pressure that was present when the breed was being developed. When a producer stops paying attention to internal parasite resistance and selects animals with no regard to that trait, weaker animals may be retained for breeding.

This lamb is the F1 generation from Gulf Coast and Suffolk parents. Photo: Linda Coffey, NCAT
Still, it is useful to know which breeds have shown parasite resistance. Incorporating one of those breeds may have almost immediate impact on internal parasite problems and will have long-term benefits. Again, the farm goals and production traits of importance must be kept in mind. Also, when using a resistant breed for crossbreeding, there will be a lot of variability in the F1 and F2 generation. (Crossing two breeds results in the F1 generation; crossing the F1 ewes with F1 rams yields the F2 generation.) See, for example, the work of J. E. Miller, who experimented with Suffolk (susceptible) and Gulf Coast Native (resistant) sheep (Miller et al., 2006). During that experiment, he found in one infection period FEC in the F2 sheep ranging from 167-149,933 eggs per gram.

St. Croix and Katahdin sheep. Photo: Joan Burke, ARS
In general, breeds with some tropical influence are thought to be more resistant to internal parasites. For example, Hampshire ewes were shown to be less resistant than St. Croix, Katahdin, and Dorper ewes (Burke and Miller, 2002). Also, Dorper lambs were less resistant than Katahdin lambs, which were less resistant than St. Croix lambs (Burke and Miller, 2004). Katahdin was more resistant than Dorper and Dorset breeds (Vanimisetti et al., 2004). Gulf Coast Native, Florida Native, St. Croix and Barbados Blackbelly are sheep that were selected in tropical areas, and they have been shown to be more resistant than Rambouillet; Hampshire; Finn-Dorset x Rambouillet; Suffolk; and Dorset x Rambouillet (summarized in Amarante and Amarante, 2003).

Gulf Coast Native sheep are resistant to internal parasites. Photo: Linda Coffey, NCAT
Some animals are not resistant to parasites but are able to produce well and remain healthy in spite of internal parasite exposure. These animals are termed “resilient” or “tolerant.” There are obvious advantages to resilient animals because they may require fewer treatments and can continue being productive under challenge. The disadvantage is that resilient animals may be spreading a lot of internal parasite eggs in their manure, thereby contaminating the farm and causing health problems for other (non-resilient and non-resistant) animals.
It can be difficult to see the difference between resistance and resilience, unless you do fecal egg counts to get a sense of the worm population within the animal and the overall challenge on the herd. A resistant animal, like a resilient one, should appear healthy and vigorous. If H. contortus (a bloodsucker) is the main problem, then both resilient and resistant animals will not be anemic, while susceptible animals with sufficient challenge will show illness, including pale membranes.
Also, on farms where there is not much challenge (not many parasite larvae present in the environment), all animals can appear resistant or resilient. The first years of having small ruminants on a farm often are trouble-free (concerning internal parasite infection), lulling the producer into a false sense of security. Unfortunately, when there is sufficient challenge to identify the resistant or resilient animals, there will be susceptible animals suffering from illness and needing deworming treatment.
The good news is that selecting animals for resistance to internal parasites seems to be sustainable. After selecting sheep lines for 10 years for high or low FEC when exposed to H. contortus, researchers challenged the sheep with both H. contortus and Trichostrongylus colubriformis. The parasites did not adapt to the resistant animals, as they can to drugs (Kemper et al., 2009). Also, as shown in this research and in others, selecting animals for resistance to one species of parasite also helps confer resistance to another (Gruner et al., 2004; Hoste and Chartier, 1998; Sreter et al., 1994; Gauly and Erhardt, 2001; Green et al., 1999; Wolf et al., 2008).
Measuring Resistance or Resilience
Measuring fecal egg counts is the most accurate way to identify animals with internal parasite resistance within a herd or flock. Resistant animals’ immune systems will not allow larvae to establish and develop into mature egg-laying adults, or will suppress the egg-laying ability of the adults that do establish. Therefore, resistant animals will not be shedding as many eggs in their feces as similarly exposed non-resistant animals.
However, there are many factors that affect fecal egg counts besides the susceptibility of the animal. These include the level of exposure (challenge), stage of production of the animals (young or lactating animals may shed more eggs), and the type of forage being grazed (consuming high-tannin forage such as sericea lespedeza causes fecal egg counts to drop dramatically). Supplementation or otherwise providing better nutrition has been shown to lower FEC (Kahn et al., 2003; Eady et al., 2003) and reduce anemia (Burke et al., 2004). Also, the parasites themselves account for some variation. Some parasites (such as Haemonchus contortus) are very prolific and will produce a lot of eggs. Other species may not; for those, a lower egg count may still mean a serious internal parasite infection. Also, internal parasites don’t lay eggs continuously and so eggs are not evenly distributed in feces. If you sample an animal twice, you will find some variation in fecal egg count even on the same day. And the number of adult worms inside the animal may not be well correlated with the fecal egg count (Saddiqi et al., 2010); immature adults and older worms produce less and males produce none.

Katahdin ewe and lambs. Photo: Margo Hale, NCAT
With all this in mind, it is clear that fecal egg counts are not a perfect tool. However, the information gained is very useful and doing fecal egg counts is the best way to assess challenge on the flock or herd and to find those animals that are harboring fewer internal parasites (Gray, 1998). Breeding decisions can be based on one or two samples if fecal egg counts are done during a time of high challenge, such as at weaning or early post-weaning for lambs, and during lactation for ewes. During those times, the animals that are resistant will stand out, and this is the time when heritability is higher (Gauly and Erhardt, 2001). Doing more than one sample improves the assessment of heritability, but this must be balanced against the cost.
Many producers do their own fecal egg counts. The process is fairly simple, and it can be expensive to have a veterinarian process samples. Also, not all veterinarians report quantitative results. There are workshops where the procedure is taught, and there are also instructions available online. See the Resources section to find links to tutorials.
The National Sheep Improvement Center (NSIP) calculates estimated breeding values (EBV) for sheep producers and breed associations. The EBV is based on progeny performance and evaluates the genetic merit of an animal for a particular trait. The Katahdin breed is currently the only U.S. breed that has EBVs for parasite resistance, using fecal egg counts from lambs at weaning and early post-weaning. Australian breeds have been calculating EBVs for parasite resistance for much longer.

This yearling dairy doe is nursing twins and may have a higher fecal egg count than an older or dry doe. Photo: Linda Coffey, NCAT
To improve a herd or flock, producers will want to consider internal parasite resistance or resilience in conjunction with other goals, such as growth, reproduction, milk production, and overall health. Also, using data such as fecal egg counts requires consideration of all the factors that influence fecal egg counts. It would not be fair to compare the fecal egg count of a dry four-year-old ewe to that of a twin four-month-old lamb or that of a yearling ewe raising twins. A single lamb that has had access to excellent pasture and creep feed will have an edge over one that has been a nursing triplet on average pasture. Be sure to compare “apples to apples” when using the fecal egg count data to select animals for breeding.
Factors Affecting Fecal Egg Counts
- Level of larval challenge affected by:
- Pasture management
- Weather
- Stocking rate (animal density)
- Species composition (types of worms)
- Worm burden
- Immune response of animal (affecting worm establishment and adult fecundity) affected by:
- Genetics
- Age
- Production stage
- Stress (including nutritional)
- Dietary factors
- Quality of pasture, especially protein levels
- Pasture species composition
- Pasture height and presence of browse or forbs
- Pasture management
- Overall quality and quantity of diet
- Selective grazing habits
- Variability of egg distribution within the fecal sample
- Diurnal patterns of egg laying
- Food transit times
- Fecal throughput and consistency
- Laboratory technique
- Collecting sample
- Preparing sample
- Counting eggs
Given all of these factors, the accuracy of fecal egg counts is improved if you take more than one sample—and you need to compare numbers within sampling time (don’t compare across seasons or years) and within groups of animals (don’t compare across ages or production stages). There is some indication that you can save effort and expense and still get a good indication of genetic merit of a sire by doing a pooled sample within a group of half-siblings.
Focusing on selecting resistant sires may be the most cost-effective and helpful approach for flock improvement (Douch et al., 1996). Sire evaluation accuracy increases with the number of offspring evaluated and the number of farms where the sire is used, as this decreases the variability caused by dam and by management. In a study conducted with Katahdin lambs where fecal egg counts were measured at 8 and 22 weeks, there were “large and significant” sire effects at both times, and these sires maintained their ranking across years, flocks, and measurement times. This emphasizes the importance of selecting good rams to improve the health of your flock (Notter et al., 2007).
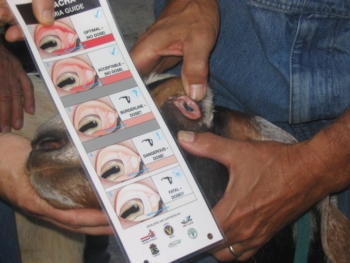
Use FAMACHA© to assess levels of anemia. Photo: Margo Hale, NCAT
Fecal egg counts provide more detailed information to guide producers in selecting animals that are not shedding as many internal parasite eggs. However, it is labor-intensive and can be costly. There is an alternative method for finding resistant or resilient animals, if Haemonchus contortus (barberpole worm, a blood-sucking parasite) is the primary parasite. The FAMACHA system was developed in South Africa as a means of assessing anemia, a symptom of infection of barberpole worm. To use this method, a trained producer simply examines the inner surface of the lower eyelid and compares the color of the membranes to the five shades of pink on the FAMACHA card. A score of 1 (bright pink) indicates no anemia, while a score of 5 (white) means severe anemia and severe infection. Producers can chart the scores of the flock or herd and record the scores on each animal every two weeks during the parasite season, and deworm only those animals that are anemic (scores of 4 and 5, or 3 if other indications, such as poor body condition, are present). In areas where barberpole worm is the main parasite, FAMACHA can serve as a quick and inexpensive way to select animals with fewer parasite problems. However, some animals can have a good FAMACHA score (brighter pink, a 1 or 2) and yet be shedding some eggs in their feces. These animals are resilient rather than resistant. Still, research has shown a good correlation with FAMACHA score, packed cell volume (PCV, a measure of anemia), and fecal egg counts where H. contortus is the main parasite in the population (Bisset et al., 2001; Kaplan et al., 2004; Burke et al., 2007; Burke and Miller, 2008).
What do you learn from FAMACHA score?
If a given animal has a FAMACHA score of 1, you can say that the animal is not anemic. But you don’t know why unless you look at more data; it could be that the animal has not been challenged by Haemonchus contortus. Or it could be that the animal has been challenged, but is resilient. Finally, it might be that the animal has been challenged but is resistant.
To decide which is true, you have to look at the rest of the flock: are any of them anemic, or are all scoring well with FAMACHA? If all are doing well (not anemic), then probably the challenge is not high enough yet to cause illness. Keep watching. And remember that many internal parasites do not cause anemia; be alert for other signs of illness, including loss of weight, animals that are lagging behind, or scours.
If some are anemic (indicating that Haemonchus is causing a problem) while others are doing well, then you have identified some animals that handle the challenge of Haemonchus. Are they resilient or resistant? A fecal egg count can help sort that out; high counts on an animal that is not anemic may indicate resilience. Very low counts point to a resistant animal. Repeated observations are necessary for more accurate decisions.
The point is that a single FAMACHA score does not really tell what is happening on a farm or even in a particular animal. Noting the condition of the whole flock or herd—and doing this over the course of the whole season—and using fecal egg counts to gain further information can help a producer understand the state of the internal parasites that reside on the farm. Charting the FAMACHA scores and observing the trend is a great help in managing the health of the flock or herd, and checking animals on a regular schedule will eventually give confidence in the ability of a particular animal to remain healthy. But one good FAMACHA score is not a reason for complacency. Use the system as it is intended for a quick, inexpensive way to diagnose animals needing treatment and, more importantly, to select the most resistant or resilient animals for breeding.
Another way to assess the health of animals (and in doing so, be able to identify more parasite-resistant animals) is called the Five Point Check© (see Table 1). This system has been taught in South Africa and is a reminder to look at the whole animal when deciding whether or not internal parasites are a problem (Bath and van Wyk, 2009). This approach helps detect the presence of internal parasites in addition to Haemonchus contortus. Many producers already do a version of this.
| Table 1. Five Point Check© | |||
| Point | What to check | Which parasites | |
| 1 | Eye | Paling of ocular membranes | Barber pole worm |
| FAMACHA© score | Liver fluke | ||
| 2 | Back | Body condition score | All |
| 3 | Rear | Dag score | Brown stomach worm |
| Fecal soiling | Hair worm | ||
| Evidence of scouring | Threadworm | ||
| Nodule worm | |||
| 4 | Jaw | Sub-mandibular edema | Barber pole worm |
| “Bottle jaw” | Liver fluke | ||
| 5 | Nose | Nasal discharge | Nasal bots |
| Source: www.sheep101.info/201/parasite.html | |||
Of course, body condition score may be low for other reasons, including poor nutrition, heavy milking, diseases such as Johne’s, or poor teeth. Nasal discharge can also occur for other reasons, and nose bots are not a problem in all regions. One additional point to make concerning “dag score”—fecal soiling, due to scouring— is that there is evidence that some animals with resistance to internal parasites have more diarrhea (scouring). It is thought that their immune response includes diarrhea as a way to shed internal parasites. Therefore, some animals that have been treated with dewormers because of this symptom are actually resistant to internal parasites (Wolf et al., 2008). Scouring also can be a result of lush pasture, or it can indicate coccidiosis. It is important to examine all the evidence when assessing animal health.
Another important piece of evidence is animal vigor. An animal that is lethargic or lagging behind the flock is likely to have some health issue, and internal parasites are often the culprit. It is a good idea to examine those animals closely and treat as needed.
How to Use This Information in Selecting Animals in Your Herd or Flock
- What resources do you have, and how much time and money can you spend
- Minimal—always record anthelmintic treatments and cull those individuals needing more than three treatments a year; don’t select ram lambs or buck kids from dams or sires that require frequent treatment or from farms that do not keep records.
- Medium—as above, but also do FAMACHA if Haemonchus contortus is a problem in your area, and keep those records. Record weights of lambs and kids. Use an index to factor in age of dam, type of birth, and days of age; retain those animals that can thrive in your system and perform well with less intervention.
- More resources and/or more motivation to improve quickly—as above, but also take fecal samples and have quantitative counts, and record those. If H. contortus is present, use FAMACHA to monitor internal parasite infection and take fecal samples during a time when animals are challenged. Taking another sample a month later can add confidence for breeding decisions. Again, remember to consider age of the animal and production stage and number of nursing progeny, or this favors single births and dams nursing singles or not lactating.
As your flock or herd improves, you can select with greater pressure; cull any animal needing two treatments a year, or one, for example. As contamination decreases on the farm, your animals should have less and less trouble with parasites and have better production.

Keeping records and selecting animals with the ability to fight off parasites is the best long-term strategy for managing internal parasites. Photo: Linda Coffey, NCAT
Encouragement
It may seem that selecting for resistance to internal parasites involves a lot of extra work. Researchers admit that it will take a lot of time to make significant progress so that a flock will be relatively free of clinical disease even under challenge. Internal parasites have many advantages in this game, including the ability to wait for the right time to become active again and infect animals or to actively breed and lay eggs so that eggs will be deposited during a favorable time of the year. Parasites are prolific and can cause enormous problems to the host in a relatively short period of time.
But research has shown that significant progress can be made and that health and production of the sheep and goats will improve as a result. Strategies for identifying sires with superior resistance do exist and can make a great difference in a flock or herd when they are employed. Selecting for resistance while keeping production traits also in mind can save a producer a lot of money and heartache as the animals themselves help fight internal parasites and remain healthier. Pasture contamination is reduced when resistant animals are present.
Ten years from now, sheep and goats could be much more resistant if producers will put time and effort into identifying and selecting the sires that are more resistant. Next year, your own flock could be more resistant than it is now. Each breeder who puts effort into selecting for this trait will benefit the business. Organic producers will benefit from having resistant stock, but so will non-organic producers because anthelmintics are not always effective and parasites have developed resistance to many of the existing drugs.
As mentioned earlier, some breeders are taking advantage of the National Sheep Improvement Program (NSIP) services to establish estimated breeding values (EBVs) for parasite resistance. This has been done in Australia with great results. The NSIP is now teaming up with Australian geneticists to strengthen the capacity of U.S. and Australian breeders to make improvements. Producers who support breeders who are using EBVs for internal parasite resistance will be voting with their dollars for a more sustainable system. It takes a concerted effort among breeders within a particular breed to develop resistant genetics.
Summary
Selecting animals with the ability to fight off internal parasites (and other diseases) is the best long-term strategy for managing internal parasite problems. There are a variety of methods accessible to the producer to help with this aspect of animal selection. Animal selection is a vital tool in improving sheep and goat herds.
Still, animal selection is not the only tool a producer will need. To have a profitable and productive enterprise, a producer will want to use all the tools, especially pasture management, because none of the other tools will be effective without good pasture management. Using as many of the tools as possible and paying attention (and spending time and money) on identifying and selecting those animals that can resist internal parasites and/or be resilient to the effects of internal parasites will pay dividends for years to come. Animal selection is a vital component of a holistic parasite management strategy.
| Internal Parasite Management | ||
| Yes | No | |
| 1. Are parasites kept at a level that does not affect animal performance? | ||
| How do you know? | ||
| How do you monitor the parasite load in your animals? | ||
| 2. What practices do you use to reduce parasite problems and avoid the use of anthelmintics? | ||
| Cull animals that get dewormed the most | ||
| Use cleaner pastures (rest pastures, cut for hay, graze cattle) | ||
| Graze diverse pastures | ||
| Reduce stocking rate | ||
| Avoid grazing pastures shorter than 3 inches | ||
| Use browse and/or forages with high condensed tannin content | ||
| Graze cattle or horses with goats or sheep | ||
| Separate classes of susceptible animals | ||
| Raise breeds and individuals with resistance to parasites | ||
| Select rams or bucks with parasite resistance | ||
| 3. What parasite control program do you use to reduce the use of anthelmintics and manage parasite loads? See www.scsrpc.org for information about these techniques. | ||
| Visual observation to detect animals with parasite problems | ||
| Use FAMACHA (see www.scsrpc.org) | ||
| Check fecal egg counts prior to and following treatment to monitor loads and check effectiveness of anthelmintics | ||
| Change class of anthelmintic once resistance is noticed | ||
| Strategic deworming just before kidding or lambing | ||
| Deworm all new animals (and check fecal egg counts seven to 10 days later to be sure there are no eggs in the feces) | ||
| Use Smart Drenching (see www.scsrpc.org) | ||
| Deworm only those animals that need it | ||
| Cull animals that need frequent deworming (more than three treatments per season for adults; less as your flock or herd gets stronger) | ||
| Other: List here: | ||
| Source: Small Ruminant Sustainability Checksheet, ATTRA | ||
References
Amarante, A.F.T., and M.R.V. Amarante. 2003. Breeding sheep for resistance to nematode infections. Journal of Animal and Veterinary Advances 2. Volume 3, p. 147–161.
Bath, G.F., and J. A. van Wyk. 2009. The Five Point Check© for targeted selective treatment of internal parasites in small ruminants. Small Ruminant Research. Volume 86, Issue 1. p. 6–13.
Bisset, S.A. and C.A. Morris. 1996. Feasibility and implications of breeding sheep for resilience to nematode challenge. International Journal for Parasitology. Vol. 26. p. 857-868.
Bisset, S.A., J.A. Van Wyk, G.F. Bath, C.A. Morris, M.O. Senson, and F.S. Malan. 2001. Phenotypic and genetic relationships amongst FAMACHA score, faecal egg count and performance data in Merino sheep exposed to Haemonchus contortus infection in South Africa. Proceeding of the 5th International Sheep Veterinary Congress. , Cape Town, South Africa.
Burke, J.M., and J.E. Miller. 2002. Relative resistance of Dorper crossbred ewes to gastrointestinal nematode infection compared with St. Croix and Katahdin ewes in the southeastern United States. Veterinary Parasitology. Vol. 109, Issues 3-4. p. 265-275.
Burke, J.M. and J.E. Miller. 2004. Relative resistance to gastrointestinal nematode parasites in Dorper, Katahdin, and St. Croix lambs under conditions encountered in the southeastern region of the United States. Small Ruminant Research. Vol. 54, Issues 1-2. p. 43-51.
Burke, J.M., Miller, J.E., Olcott, D.D., Olcott, B.M. and Terrill, T.H. 2004. Effect of copper oxide wire particles dosage and feed supplement level on Haemonchus contortus infection in lambs. Veterinary Parasitology. Vol. 123. p. 235–243.
Burke, J.M. and J.E. Miller. 2008. Use of FAMACHA system to evaluate gastrointestinal nematode resistance/resilience in offspring of stud rams. Veterinary Parasitology. Vol. 153. p. 85-92.
Douch, P.G.C., R.S. Green, C.A. Morris, J.C. McEwan, and R.G. Windon. 1996. Phenotypic markers for selection of nematode-resistant sheep. International Journal for Parasitology. Vol. 26, Issues 8-9. p. 899-911.
Eady, S.J., R.R. Woolaston, and I.A. Barger. 2003. Comparison of genetic and nongenetic strategies for control of gastrointestinal nematodes of sheep. Livestock Production Science. Vol. 81, Issue 1. p. 11-23.
Gauly, M. and G. Erhardt. 2001. Genetic resistance to gastrointestinal nematode parasites in Rhön sheep following natural infection. Veterinary Parasitology. Vol. 102, Issue 3. p. 253-259.
Gray, G.D. 1997. The use of genetically resistant sheep to control nematode parasitism. Veterinary Parasitology. Vol. 72, Issues 3-4. p. 345-366.
Green, R.S, C.A. Morris, P.G.C. Douch, M. Wheeler, C.J. West, and S.M. Hickey. 1999. Means and heritabilities of concentrations of antibody to Trichostrongylus colubriformis and other nematode parasites in lambs from three to seventeen months of age. Livestock Production Science. Vol. 58, Issue 2. p. 129-135.
Gruner, L., J. Bouix, J.C. Brunel. 2004. High genetic correlation between resistance to Haemonchus contortus and to Trichostrongylus colubriformis in INRA 401 sheep. Veterinary Parasitology. Vol. 119, Issue 1. p. 51-58.
Hoste, H. and C. Chartier. 1993. Comparison of the effects on milk production of concurrent infection with Haemonchus contortus and Trichostrongylus colubriformis in high- and low-producing dairy goats. American Journal of Veterinary Research. Vol. 54, No. 11. P. 1888–1893.
Hoste, H. and C. Chartier. 1998. Response to challenge infection with Haemonchus contortus and Trichostrongylus colubriformis in dairy goats. Consequences on milk production. Veterinary Parasitology. Vol. 74, Issue 1. p. 43-54.
Kahn, L.P., M.R. Knox, S.W. Walkden-Brown, and J.M. Lea. 2003. Regulation of the resistance to nematode parasites of single- and twin-bearing Merino ewes through nutrition and genetic selection. Veterinary Parasitology. Vol. 114, Issue 1. p. 15-31.
Kaplan, R.M., J.M. Burke, T.H. Terrill, J.E. Miller, W.R. Getz, S. Mobini, E. Valencia, M.J. Williamson, M. Larsen, and A.F. Vatta. 2004. Validation of the FAMACHA eye color chart for detecting clinical anemia in sheep and goats on farms in the southern United States. Veterinary Parasitology. Vol. 123, Issues 1-2. p. 105-120.
Kemper, K.E., R.L. Elwin, S.C. Bishop, M.E. Goddard, and R.R. Woolaston. 2009. Haemonchus contortus and Trichostrongylus colubriformis did not adapt to long-term exposure to sheep that were genetically resistant or susceptible to nematode infections. International Journal for Parasitology. Vol. 39, Issue 5. p. 607-614.
Miller, J.E., S.C. Bishop, N.E. Cockett, and R.A. McGraw. 2006. Segregation of natural and experimental gastrointestinal nematode infection in F2 progeny of susceptible Suffolk and resistant Gulf Coast Native sheep and its usefulness in assessment of genetic variation. Veterinary Parasitology. Vol. 140, Issues 1-2. p. 83-89.
Notter, David, J. Morgan, and B. Vanimisetti. 2007. Tools for Genetic Improvement of Parasite Resistance: Development of a Fecal Egg Count EPD. Katahdin NSIP Notebook.
Saddiqi, Hafiz, H.A. Abubaker, Z. Iqbal, M.N. Khan, and G. Muhammad. 2010. Comparative resistance of sheep breeds to Haemonchus contortus in a natural pasture infection. International Journal of Agriculture and Biology. Vol. 12, No. 5. p. 739-743.
Sréter, T., T. Kassa,i and E. Takács. 1994. The heritability and specificity of responsiveness to infection with Haemonchus contortus in sheep. International Journal for Parasitology. Vol. 24, Issue 6. p. 871-876.
Torres-Acosta, J.F.J. and H. Hoste. 2008. Alternative or improved methods to limit gastro-intestinal parasitism in grazing sheep and goats. Small Ruminant Research. Vol. 77, Issues 2-3. p. 159-173.
Vagenas, D., F. Jackson, A.J.F. Russel, M. Merchant, I.A. Wright, and S.C. Bishop, 2002. Genetic control of resistance to gastro-intestinal parasites in crossbred cashmere-producing goats: responses to selection, genetic parameters and relationships with production traits. Animal Science. Vol. 74. p. 199-208.
Vanimisetti, H.B., S.P. Greiner, A.M. Zajac, and D.R. Notter. 2004. Performance of hair sheep composite breeds; resistance of lambs to Haemonchus contortus. Journal of Animal Science. Vol. 82, No. 2. p. 595-604.
Woolaston, R.R. 1992. Selection of merino sheep for increased and decreased resistance to Haemonchus contortus: peri-parturient effects on faecal egg counts. International Journal for Parasitology. Vol. 22, No. 7. p. 947-953.
Wolf, B.T., K. Howells, C. Nakiclny, W. Haresign, R.M. Lewis, Ol. Davies and M.H. Davies. 2008. Genetic parameters for strongyle and Nematodirus faecal egg counts in lambs and their relationships with performance traits. Livestock Science. Vol. 113, Issues 2-3. p. 209-217.
Further Resources
Sustainable Agriculture Research and Education (SARE)
The SARE website holds many research reports of interest to sheep and goat producers. To access these reports, go to the homepage, click on “project reports” and then search “internal parasite” to bring up a list of reports that can be informative on this subject. There is a PowerPoint presentation on the subject of selecting animals for internal parasite resistance that is very informative and interesting. The presentation illustrates many important concepts of selecting animals for internal parasite resistance. Also see the report on that Farmer/Rancher SARE project, FNC05-583.
American Consortium for Small Ruminant Parasite Control (ACSRPC)
ACSRPC was formerly known as the Southern Consortium for Small Ruminant Parasite Control (SCSRPC) and provides up-to-date scientific research and recommendations for producers. There is a six-part series of articles written for producers and previously published in Goat World, as well as other articles, including information about FAMACHA and Smart Drenching.
A summary of SARE-funded work done by the SCSRPC is collected in this article.
Langston University
Langston University’s website includes two tutorials for doing fecal egg counts. The information is slightly different in these presentations. Also see the chapter in the web-based training manual for more complete information about internal and external parasite control.
Maryland Small Ruminant Page
Susan Schoenian is an educator with the University of Maryland Cooperative Extension Service. She has generously shared information with the world through this website. She also has posted some excellent presentations at Slideshare, including some about integrated parasite management. These presentations are very helpful and will add to understanding of the problem and solutions. Access them from the main website.
Tools for Managing Internal Parasites in Small Ruminants: Animal Selection
By Linda Coffey, NCAT Agriculture Specialist
Published 2012
©NCAT
IP400
Slot 394
Version 051612
This publication is produced by the National Center for Appropriate Technology through the ATTRA Sustainable Agriculture program, under a cooperative agreement with USDA Rural Development. This publication was also made possible in part by funding the USDA, NIFA, OREI (Award No. 2010-51300-21641); and the Southern Region Sustainable Agriculture Research. ATTRA.NCAT.ORG.

