Dung Beetle Benefits in the Pasture Ecosystem
By Michelle L. Thomas, NCAT Agriculture Intern, and Omar Rodriguez, NCAT Sustainable Agriculture Specialist
Abstract
This publication discusses the important and interesting role of the dung beetle, including the benefits it provides in the pasture ecosystem. It contains information on dung beetle farming and relates an author’s experiences observing dung beetles.
Contents
Introduction
Appearance and Behavior
Importing New Species
Benefits to the Pasture System
Management
References
Further Resources
Appendix A: A Personal Note from Author Michelle Thomas
Appendix B: Dung Beetle Life Cycle Viewing Chamber
Introduction
Dung beetles play an important and remarkable role in the pasture ecosystem. While they feed on manure and use it to provide housing and food for their young, they are also improving nutrient cycling, soil structure, and forage growth. Dung beetles are significant enough to the processes of pest reduction and manure and nutrient recycling that they are well deserving of the pasture manager’s attention.
Dung beetles belong to the zoological order Coleoptera and family Scarabaeidae, commonly known as scarab beetles. Of the more than 90 species in the United States, less than a dozen are significant in dung burial. Three behavioral groups of the beetles are relevant to manure recycling. Probably the best-known group are the “tumble bugs” or “rollers” (e.g., the species Canthon pilularius). The male and female pair of this group roll a ball of dung (brood ball) away from a manure pile in order to bury it.
Another group are the “tunnelers.” Tunnelers like Onthophagus gazella are especially desirable due to their habit of drawing manure down from the soil surface into the ground below the dung pat (Kaufman and Wood, 2012). Piles of soil next to the dung pat are indicators of tunneler-type beetle activity. Collectively, tunnelers and tumblers are classified as “nesters” because of their behavior in preparing a home for their young. The third group of beetles that use dung are the “dwellers.” Most dwellers belong to the subfamily Aphodiidae. They live within the manure pat, engage in little to no digging, and generally do not form brood balls.
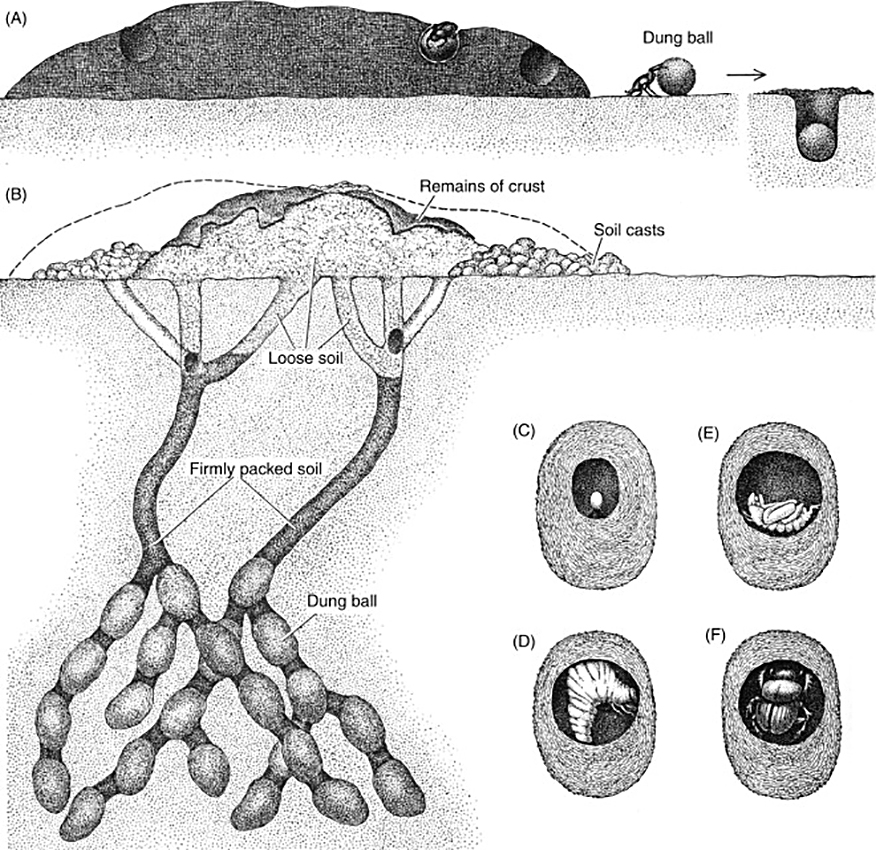
Cowpats are used in different ways by different dung beetles. One group cuts bits of dung out of the pat, forms them into balls, and rolls them away for burial in a shallow pit (A). Most species of dung beetles form their nests in tunnels excavated below the cowpat (B). A male and female beetle dig the tunnel and carry dung down into it. The female then forms a ball (as in the case of Onthophagus gazella shown here), lays an egg in it, and closes the ball (C). The tunnels are back-filled with firmly packed soil. Loose soil fills the upper parts of the tunnels and is left on the surface, along with some remains of the cowpat. When the larva hatches (D), it feeds on the dung. After passing through the pupal stage (E) a young adult (F) emerges and makes its way to the surface. Source: Fincher, G.T. and P.B. Morgan
Appearance and Behavior
Dung beetles range in size from 2 mm (0.1 inch) to 60 mm (2.5 inches). The front legs usually have serrated edges, used for powerful digging. Colors range from black to brown to red, and can have a metallic appearance. Males often have one or two horns. Scarabs are distinguished from other beetles by the appearance of their antennae, which are segmented and end with a plate-like oval club of three to seven expansible leaves. These lobes create a large surface area for detecting odors. Look for these specialized antennae with a magnifying glass.
Adult beetles are drawn to manure by odor. Many are species-specific, in that they prefer a certain type of animal manure. They will fly as far as 10 miles in search of just the right dung, and can attack dung pats within seconds after they drop. Some species will even hitch a ride near the tails of animals in anticipation of a deposit. Once drawn by the odor, the adults use the liquid contents of the manure for their nourishment. Dr. Patricia Richardson, formerly Research Associate at the University of Texas, memorably refers to this as a “dung slurpie.”
If they are a nesting species, the pair then goes to work on forming a brood ball out of the dung, which contains a large amount of roughage. The pair continue to work as a team to bury the ball. The female, which typically has shorter, thicker legs, digs while the male helps haul the soil from the tunnel. The female lays one egg in each ball. She then seals the brood ball, seals the tunnel, and begins the process again if she is of a species that lays several eggs.
In about a week, the egg hatches within the brood ball. The larva feasts on the interior contents of the ball, eating about 40% to 50%, and sealing the interior with its own excrement along the way. This leads to a totally enclosed, protected environment. The larva does not have to compete with others for a food source and is also protected from predators outside the brood ball. If the integrity of the brood ball is destroyed, the larva will die. Under ideal environmental conditions, the larva will pupate at an average of three weeks. A young-adult beetle emerges, eats its way out of the brood ball, forms a new tunnel to exit through, and goes on its way in search of fresh manure. The newly emerged beetles will breed two weeks later, with a complete generation taking six weeks under ideal environmental conditions (Richardson and Richardson, 2000).
Soil moisture level is crucial to many species, as breeding and dung burial decrease during dry periods. In dry weather, the young adults emerge from the brood ball but remain within the soil, waiting for rain. As with most beetles, activity decreases during the coldest months. The larvae remain viable deep within the soil, waiting for environmental cues such as rainfall and temperature to prompt their emergence.
Some broad-spectrum insecticides can have detrimental effects on beneficial insects like dung beetles. Farmers interested in funding to help transition to alternative pest control methods can access the USDA’s Conservation Stewardship Program (CSP). The alternative pest control practice can be found under Integrated Pest Management practice 595.
NRCS accepts applications for the Conservation Stewardship Program (CSP) at any time throughout the year. NRCS sets specific deadlines for ranking and funding opportunities. Contact your local NRCS field office for more information.
Importing New Species
Dr. Truman Fincher (retired) directed the dung beetle research program at the USDA-ARS Food Animal Protection Research Laboratory at College Station, Texas, until 1998. His research was directed at importing and introducing dung beetle species that would complement and not compete with native populations, in order help balance U.S. pasture ecosystems. According to Fincher, the beetles in the United States have not been able to keep up with our increased livestock production and manure waste. Increased fertilizer use and higher-producing forage varieties have boosted forage yields, in turn increasing the animal carrying capacity per unit of pasture. Also, widespread use of insecticides, herbicides, fungicides, and anthelmintics can drastically reduce dung beetle populations (Habeck et al., 1990).
Extensive introduction of dung beetle species has taken place across the globe. The selection of appropriate species has led to interesting research that has yielded deep insights into the habits and abilities of many species.
Some are nighttime flyers and navigate by the dim glow of the Milky Way, while some prefer to do their work during the day, and some prefer older manure to very fresh. If several species are working together, some may bury the brood ball close to the manure pat, some farther away, some shallow, and some deep (Fincher, 1981). Much research has been done in efforts to understand which species to introduce to a particular environment. The bulk of species introduction in the United States took place during the 1970s, particularly across the southern and western states. Some of these species took it upon themselves to spread to other states where the release of non-native species was not planned (Kaufman and Wood, 2012). It seems that state boundaries could do nothing to keep the dung beetles’ tibial spurs off of the sweet scat.
Benefits to the Pasture System

The Afro-Asian dung beetle, Onthophagus gazella, has been successfully established in the southern tier of states, from California to South Carolina. Photo: ipmimages.org
Dung beetles’ benefits to livestock and the pasture environment should outweigh any reservations we may have about their dietary choices. In addition to aiding in the processing of manure on the soil surface, beetles also help to reduce survival rates of ruminant-parasitic nematodes and flies. For example, manure is the breeding ground and incubator for horn flies (Haematobia irritans) and face flies (Musca autumnalis), two economically important pests of cattle. A single manure pat can generate 60 to 80 horn fly adults if protected from insect predators and competitors such as dung beetles. As beetles feed, they compete with the fly larvae for food and physically damage the flies’ eggs. Fly populations have been shown to decrease significantly in areas with dung beetle activity. Dr. George Bornemissza found that 95% fewer horn flies emerged from cowpats inhabited by Onthophagus gazella than from pats where beetles were excluded (Knutson, 2000).
Dung beetles are also reported to be effective biological control agents for gastrointestinal parasites of livestock. The eggs of most gastrointestinal parasites pass out in the feces of the host. The eggs then hatch into free-living larvae and develop to the infective stage. They then migrate onto grass, where they can be ingested by grazing animals, and complete their life cycle within the animal. If the manure/egg incubator is removed by beetles, the eggs perish and the life cycle of the parasite is broken.
On a pasture-management level, dung pat removal is beneficial for forage availability. Most ruminants will not graze close to their own species’ manure pats. Research has shown that the forage is palatable, but avoided because of the dung pile. Consequently, cattle manure deposits can reduce available acreage by as much as 5% to 10%. By completely and quickly removing the manure, beetles can significantly enhance grazing efficiency.
The tunneling behavior of beetles increases the soil’s capacity to absorb and hold water, and their dung-handling activities enhance soil nutrient cycling. An adequate population and mix of species can remove a complete dung pile from the surface within 24 hours. As the adult beetles use the liquid component for nourishment and the roughage for the brood balls, the dung pat quickly disappears. If manure is left on the surface, up to 80% of manure nitrogen is lost through volatilization; by quickly incorporating manure into the soil, beetles make more of this nitrogen available for plant use and limits volatilization to 5% to 15%. The larvae use only 40% to 50% of the brood ball before pupating, leaving behind the remainder of this nutrient-rich organic matter for soil microbes, fungi, and bacteria to use in creating humus (Richardson and Richardson, 1999).
Management
Resorting to the use of pesticides as a primary means of solving issues of pest stress on cattle can place the ranch manager in a position of constant recovery. Exposing cattle to repeated applications of insecticides and nematicides has been shown to encourage resistance in target species. Additionally, these treatments can negatively impact any population of dung beetles that feed on treated cattle (Oyarzun et al., 2008; Lumaret et al., 1993). Many of the chemicals used to treat parasites are broad-spectrum agents used to target a variety of species, and these can persist in the feces of treated animals. The presence of antiparasitics in livestock feces can cause increased mortality in non-target organisms (Lumaret et al., 2002).
Dung beetle larvae are susceptible to some insecticides used for fly and internal parasite control for cattle. Some drugs belonging to the group known as Anthemintics (including coumaphos, dichlorovos, phenothiazine, piperazine, synthetic pyrethroids, and macrocyclic lactones) can be highly toxic to insects that depend on dung for breeding or as a food source. Other treatments for parasites and other pests may have little to no effect on beetles. These include chemicals belonging to the benzimidazole and levamisole/morantel groups (Lumaret et al., 2002).
Ivermectin (Ivomec and Doramectin) is one of the more studied chemicals in its effect on secondary organisms. It can be administered as an injection, pour-on, or bolus; each has a greater concentration than the previous version, with the bolus releasing up to 90% of the given dose (Lumaret et al., 2002). When the injection method was used at the recommended dose, it reduced survival of the young of two species for one to two weeks in a study done by Dr. Fincher. Ivermectin pour-on reduced survival of the larvae for one to three weeks. Most detrimental was Ivermectin administered as a bolus, with effects lasting up to 20 weeks. Discontinuing use of this type of insecticide, or using it only when necessary, will help increase your population of dung beetles.
Specific chemicals aside, one must consider that any product designed to harm, limit, or kill would have some impact on the ecosystem in general, and should be used judiciously. This might include eliminating year-round treatment and using more selective or alternative treatment methods. Backrubbers, ear tags, and the occasional use of insecticide dusts and sprays are alternatives for filth-fly control that have little or no effect on dung beetles (Knutson, 2000). Another option is to treat cattle during the coolest months of the year, as the beetles and larvae are inactive at those times. Better yet, before treating your animals for internal parasites, take a fecal sample to your veterinarian. An egg count can help determine parasite load and whether the symptoms you may be seeing in the form of low gains, weight loss, unthriftiness, etc., are truly being caused by parasites.
In order to establish a baseline, observe the length of time it takes for the manure pats to disappear in your pasture and look for hole formation in the surface of the manure pats. If the pats remain intact for more than a few days, chances are your beetle population is low to non-existent. Management is the key to increasing the number and variety of beetles and other beneficial insects.
Controlled grazing systems increase dung beetle populations and varieties by concentrating the manure in smaller areas, thus reducing the time beetles must spend in search of food. Grazing cycles that match the reproductive cycle of the beetles are favorable, as cattle return to grazing cells at the same time that new adults are emerging from the soil. For more information on controlled grazing systems, refer to the ATTRA publications Rotational Grazing and Sustainable Pasture Management.
Dung beetles are just one small part of the pasture ecosystem, but they are too important to ignore. To summarize the dung beetle benefits highlighted by Dr. Fincher:
-
- Increased pasture yields resulting from the incorporation of organic matter into the soil, with an increase in soil friability, aeration, and water-holding capacity
- Reduced populations of insect pests that breed in animal feces
- Prevention of pasture surface pollution
- Reduced animal diseases, through removing contaminated feces from pasture surfaces
- Nutrients returned to the soil that would otherwise be tied up in fecal deposits and unavailable to pasture grasses
- Increased effective grazing of pasture areas covered by feces
- Reduced nitrogen loss in livestock feces
References
Behrens, Patricia W. 1994. Dung beetles: Beetlemania in action. Acres U.S.A., October. Vol. 24, No. 10. p. 10-12.
Fincher, G.T. 1981. The potential value of dung beetles in pasture ecosystems. Journal of the Georgia Entomological Society. Vol. 16. 1st Supplement. p. 316−333.
Habeck, D.H., F.D. Bennett, and J.H. Frank (eds.) 1990. Classical biological control in the Southern United States. Southern Cooperative Series Bulletin No. 355.
Kaufman, Phillip E. and Lois A. Wood. 2012. Indigenous and exotic dung beetles (Coleoptera: Scarabadae and Geotrupidae) collected in Florida cattle pastures. Annals of the Entomological Society of America. Vol. 105, No. 2. p. 225-231.
Knutson, Allen. 2000. Dung beetles: Biological control agents of horn flies. Texas Biological Control News. Winter. Texas Agricultural Extension Service. The Texas A&M University System.
Lumaret, Jean-Pierre, Eduardo Galante, C. Lumbreras, J. Mena, Michel Bertrand, J. Bernal, J. Cooper, Nassera Kadiri, and Deirdre Crowe. 1993. Field effects of Ivermectin residues on dung beetles. The Journal of Applied Ecology. Vol. 30. No. 3. p. 428.
Lumaret, Jean-Pierre, Faiek Errouissi. 2002. Use of anthemintics and evaluation of risks for the non target fauna of pastures. EDP Sciences. Vol. 33, No. 5. p. 457-562.
Oyarzun, Maria, Andres Quiroz, and Michael Birkett. 2008. Insecticide resistance in the horn fly: Alternative control strategies. Medical and Veterinary Entomology. Vol. 22, No. 3. p. 188-202.
Richardson, Patricia Q. and R.H. (Dick) Richardson. September 1999. Factsheet: Dung beetles (Work for free, love their work).
Richardson, Patricia Q. and R.H. (Dick) Richardson. 2000. Dung beetles improve the soil community (Texas/Oklahoma). Ecological Restoration. Summer. Vol. 18, No. 2. p. 116-117.
Further Resources
Biological Control of Insect Pests: Insects in Cattle Dung. 2001. By Kevin Floate. Lethbridge Research Centre. Agriculture and Agri-Food Canada Research Branch.
The Indomirable dung beetle plays a key role in parasite regulation. 2017. By Janice Cessna. BioScience. Vol. 67, No. 6. p. 583-584.
Lifestyle and host defense mechanisms of the dung beetle, Euoniticellus intermedius: the toll signaling pathway. 2013. By R. Hull, M. Alaouna, L. Khanyile, M. Byrne, and M. Ntwasa. 2013. Journal of Insect Science. Vol. 13. p. 108.
Sustained parasiticide use in cattle farming affects dung beetle functional assemblages. 2018. By Bryony Sands and Richard Wall. Agriculture, Ecosystems & Environment. Vol. 265. p. 226-235.
Appendix A: A Personal Note from Author Michelle Thomas
My interest in this research area was sparked by observations made during a local grazing group’s pasture walks, held monthly in the Northwest Arkansas area. While walking through the pastures, participants had to carefully watch their step to avoid those proverbial “pats.” As the warm spring days arrived, we noticed holes on top of the manure pats, and began to investigate further. Seeing various small beetles, spiders, flies, gnats, and other insects led to more investigation.
Some in the group were more investigative than others, using pocketknives and sticks to plow into the manure. We found dry, hard shells with holes on the outside, and tunnels with moisture underneath. Some of the dung pats had been more thoroughly excavated with nothing left except for hollow interiors and outer shells. Many pats were spread out, with only a bit of roughage left behind. Several had piles of soil next to the edge of the pat. Having learned about dung beetles and their benefits from an ATTRA Specialist, the group had some ideas about what we were looking at. And as usual, we also had more questions. My curiosity piqued; I began to research the subject during my summer internship. I have since had the opportunity of watching the seasonal changes on the dung scene from late spring, through summer, and into early fall.
Research in the scientific literature was also interesting, but I finally turned to a few experts for the benefit of their applied knowledge. Dr. Patricia Richardson has written several publications on this topic, with a humorous style I admire. When I came across mention of a dung beetle “farm” used at a workshop in Texas, I decided to try to replicate it for myself. Dr. Richardson was very helpful and provided construction details.
Next, I needed the “workhorse” of all the tunneler dung beetles, the Onthophagus gazella. Again I called on Dr. Richardson for advice on how to locate them near my home in the Arkansas River Valley. She suggested watching at dusk and at dawn, as they are nighttime flyers. For several evenings and early mornings I followed her suggestions, to no avail. (I did see three beautiful rainbow scarabs around a pat by flashlight late one evening.) Frustrated, I went to Plan B: I scooped up an entire manure pat with the telltale sign of tunneler activity, a fresh soil mound next to it, and bagged and froze it. I dissected the pat the next afternoon, sorting out beetles by size and appearance into separate containers, and made a trip to the University of Arkansas Entomology Museum, where Dr. Jeffrey Barnes identified my beetles for me. To my utter dismay (devastation may be a better word), there were no Scarabs, or “true dung beetles.” Most of my specimens were of the Histeridae family, which is another very beneficial beetle, but not what I was looking for. Finally I turned to Oklahoma cattleman Walt Davis, who graciously sent several of the gazella beetles to me by mail.
I filled the “farm” with sandy soil from the river bottom, and put fresh cattle manure on top. The looming challenge now was to distinguish the males from the females, in order to place two or three pairs into the farm. With Dr. Richardson’s notes close at hand, I placed one beetle into a white coffee cup for close viewing. The front legs were serrated as she described, and the antennae had little lobes on the end. Males have two small horns that lie toward the back and are a little difficult to see at first. The females have shorter, thicker legs than the males, and no horns. (I must admit I have become quick at sex identification of these creatures, which is alarmingly rewarding.) I placed two pairs into the farm and waited.
Within three days, we began to see tunnels forming. I added another pair and the brood balls became visible within a few more days. I cannot adequately describe my excitement. After two weeks, at least 38 brood balls were present, indicating time to entice the parents out of the nest. Dr. Richardson suggested “starving” them out for a few days, then luring them into a new, fresh pile of manure. The process worked very well.
At the time of writing, I am watching the brood balls for movement and hatching, approximately four weeks after their burial. I have seen two larvae moving and eating, and hope they will consider the sheet of Plexiglas an integral part of the brood ball for later pupation. The weather, however, will have an effect, since it is cooling off below 55° F at night. This will slow their activity, and, from my understanding, may even arrest their emergence until the warm spring evenings and rainfall begin. Even so, this dung beetle farm can be used for presentations and educational opportunities for several months and that is my intention.
One last note of excitement over this project: I located several dung beetles I believe to be gazellas while cleaning the poultry pens at our county fair in September, after a long, much-needed rain. Moisture is critical to their activity, and they showed up when and where I least expected! We have since found these tunnelers on our own farm as well, and they are most welcome to stay as long as they will.
 Appendix B: Dung Beetle Life Cycle Viewing Chamber
Appendix B: Dung Beetle Life Cycle Viewing Chamber
You can easily build your own dung beetle farm for observation of burrows, brood balls, larvae, etc. This would make a great school or 4-H project for the kids. The chamber consists of two Plexiglas sides with a ½” space between them held in a wooden frame, with a viewing area (per side) of about 24” wide by 20” tall. Information provided by Dr. G. Truman Fincher via Dr. Patricia Richardson.
Lumber needed (use treated lumber):
-
-
- Bottom: 2” x 4” x 31” long. Cut a 7/8”wide, ½” deep center groove down the entire length of the board.
- Sides: make two, 2” x 2” x 21” long. Again, cut a generous. 7/8”wide, ½” deep center groove the entire length of the board. At the bottom end of each side piece, cut the board to leave a ½”deep, 7/8” wide tongue to fit into the groove in the bottom piece.
- Braces: make two (2” x 4”) On the outside of each side piece is a wedge-shaped brace about 4” tall, glued to the side and screwed to the bottom.
- Top: 1” x 2” x 20” long. Cut a 7/8” wide, ¼”deep center groove the entire length of the board. Make a 16” long cut (the thickness of the saw blade) through the board, in the center of the groove and the middle of the board’s length—this is the air slit.
- Plexiglas needed: 2 viewing sides: 3/16” thick, 25” wide x 21” tall
- 2 end strips: ½” thick, ½” wide x 20.5” tall
- 1 bottom strip: ½” thick, ½” wide x 25” long
- 3 support circles (or squares, or triangles): ½” thick, about the diameter of a quarter, to keep the viewing sides from bowing in or out
Glue all strips and circles to one of the Plexiglas viewing sides. Place one circle in the center, about 16” from the bottom. Place the other two about 6” in from either side, and 8” up from the bottom.
When the chamber is assembled, drill a hole through each support circle (in through one Plexiglas side and out the other). Secure with bolts and nuts. Glue and screw wood frame pieces into place.
Add sandy loam soil up to about 7” from the top, fresh cow manure (big blob piled in middle), and two or three male/female pairs of adult dung beetles. Keep at warm temperature (they like 85° F). They should begin to burrow and make brood balls within a day or two. Add more fresh manure as needed. Remove the adult dung beetles in a week to 10 days (withhold fresh manure for a while, then lure them into a bucket of fresh). Provide 14 hours of light, 10 of darkness.
-
Dung Beetle Benefits in the Pasture Ecosystem
By Michelle L. Thomas, NCAT Agriculture Intern
October 2001
Updated by Omar Rodriguez, NCAT Sustainable Agriculture Specialist
June 2020
© NCAT
IP593
Slot 171
Version 062520
This publication is produced by the National Center for Appropriate Technology through the ATTRA Sustainable Agriculture program, under a cooperative agreement with USDA Rural Development. ATTRA.NCAT.ORG.


