Tools for Managing Internal Parasites in Small Ruminants: Pasture Management
By Linda Coffey and Margo Hale, NCAT Agriculture Specialists
Abstract
Proper pasture management can reduce the number of parasites ingested by sheep and goats, keeping parasite burdens low. This publication discusses techniques for managing parasites on the pasture and for increasing grazing animals’ resistance to parasites through improved nutrition. Pasture management is a vital component of a holistic parasite management strategy.
Contents
Introduction: The Internal Parasite Problem
Parasite Life Cycle and What Affects It
Grazing Animals
Pastures
Pasture Management
Summary
Pasture Management Assessment Sheets
References
Further Resources
Introduction: The Internal Parasite Problem
Internal parasite management, especially of Haemonchus contortus (barber pole worm, stomach worm), is a primary concern for the majority of sheep and goat producers. A severe infection of barber pole worm causes anemia, reduced animal production, bottle jaw, and-if not treated-death of infected sheep and goats.
Mature parasites breed inside the host and produce eggs that pass through the host and are shed in the feces. After the eggs pass out of the host, they hatch into larvae in the fecal pellet. Warm, moist conditions encourage hatching and the development of infective larvae. The larvae need moisture, such as dew or rain, to break open the fecal pellet. The infective larvae migrate out of the feces and up blades of grass (usually one to three inches, though they may go higher). When an animal grazes, it may take in parasite larvae along with the grass, resulting in infection. Numbers of infective larvae on the pasture increase over time when environmental conditions are favorable (wet, warm) and grazing animals are present to complete the cycle.
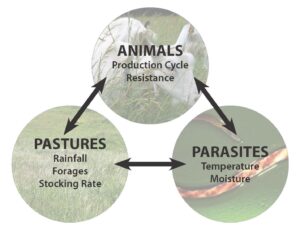
Figure 1. Managing internal parasites is possible when producers understand the interactions between pastures, animals, and parasites, and the factors affecting each. Graphic: Robyn Metzger, NCAT
The parasites live either in a grazing animal or on a pasture. For a number of years, the main strategy for managing parasites was to attack them inside the animal by treating with anthelmintics (dewormers). Parasites are now developing resistance to all commercially available dewormers. Dewormer resistance is the ability of worms in a population to survive drug treatment of the animal at the standard prescribed dosage. Over-use of dewormers (frequent deworming and treating all animals regardless of need) has resulted in dewormer resistance, and as a consequence most available dewormers are now ineffective. Producers cannot rely on dewormers alone to control internal parasites, so it is important to use several tools to manage them.
Pasture management is a fundamental tool in managing internal parasites. Proper pasture management can reduce the number of parasites ingested by sheep and goats, keeping parasite burdens low. Pasture management is also essential for providing good nutrition to the animals, which helps them resist and tolerate some internal parasites and further protects animal health. Pasture management is a low-cost tool that can be implemented immediately in a parasite-management approach (assuming you already have fencing). This publication discusses techniques for managing the parasites on the pasture and for increasing grazing animals’ resistance to parasites through improved nutrition.
Pastures, animals, and parasites all interact (see Figure 1) and are all affected by the weather, rainfall, time of year, and natural life cycles. Each species of forage, animal, and parasite may respond differently and require a different strategy for management. Therefore, in this paper we will discuss concepts and give as many specifics as possible, but there will not be a “recipe” with a guaranteed outcome. Instead, you will be armed with information to help you manage your farm to avoid severe internal parasitism. Understanding the interrelationships will help. The following sections explain factors affecting parasites, animals, and pastures and present techniques to help lessen risk to animal health.
Parasite Life Cycle and What Affects It
Factors:
Temperature
Moisture
Time
Season
Animals and soil organisms
Plant compounds
Effective anthelmintics
In order to manage internal parasites effectively, it is important to understand the factors affecting the parasite life cycle. Haemonchus contortus worms live in the abomasum and lay large numbers of eggs; one female can lay 5,000 to 10,000 eggs per day (Gordon, 1967). Other internal parasites reside in the intestines and also produce eggs. The eggs are passed in the manure onto pasture. When the weather is warm enough, those eggs on pasture will develop into larvae, which develop in stages called L1, L2, and L3. Once they reach the third stage (L3), they are infective larvae that “migrate” onto grass blades when rain or dew allow (O’Connor et al., 2007; Santos et al., 2012; Silva et al., 2008; Amaradosa et al., 2010). A heavy rain can splash the larvae some distance away from the manure in vertical and horizontal directions. Some larvae will go into the soil; that makes a “reservoir” that will protect the larvae from weather extremes (Leathwick et al., 2011). Several researchers have studied the vertical migration (how high on the grass blade the larvae are found) and the results are discouraging: larvae may be found at the top of the grass blades, more than 20 cm high (about eight inches) (Amaradosa et al., 2010; Gazda et al., 2009; Santos et al., 2012; Silva et al., 2008). However, most of the larvae are usually found near the base of the plant, especially during dry periods.
This means that controlling grazing so that animals do not graze too close to the ground will help reduce infection, though it won’t completely prevent it. Some larvae will be ingested by the animals when they are grazing.
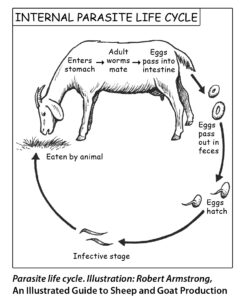 The larvae that are consumed may become established and mature inside the animal, and the cycle repeats. The eggs hatch and the larvae develop quickly in warm, moist conditions. If the pasture is receiving one inch of precipitation per month, that creates an environment sufficient for Haemonchus contortus (Stromberg, 1997). Moisture encourages egg hatching and larval development, while long dry periods cause dessication (drying) and death. A single, heavy rainfall soon after the eggs (in the manure) land on pasture results in more eggs and larvae surviving, and there are more viable eggs in goat manure that has been rained on within four days than in manure that has been dry for eight days or longer (O’Connor et al., 2007). Haemonchus contortus are not very active at temperatures of 50° F or less. They hatch and develop best at a range of 86-95°F; July to September in Kentucky is Haemonchus season (Hutchens and Chappell, 2004), while animals can be affected in April and May further south. Nematodirus, on the other hand, will become active whenever the temperature is over 50° F, so is a problem in early spring (Younie et al., 2004) and survives a long time on pasture.
The larvae that are consumed may become established and mature inside the animal, and the cycle repeats. The eggs hatch and the larvae develop quickly in warm, moist conditions. If the pasture is receiving one inch of precipitation per month, that creates an environment sufficient for Haemonchus contortus (Stromberg, 1997). Moisture encourages egg hatching and larval development, while long dry periods cause dessication (drying) and death. A single, heavy rainfall soon after the eggs (in the manure) land on pasture results in more eggs and larvae surviving, and there are more viable eggs in goat manure that has been rained on within four days than in manure that has been dry for eight days or longer (O’Connor et al., 2007). Haemonchus contortus are not very active at temperatures of 50° F or less. They hatch and develop best at a range of 86-95°F; July to September in Kentucky is Haemonchus season (Hutchens and Chappell, 2004), while animals can be affected in April and May further south. Nematodirus, on the other hand, will become active whenever the temperature is over 50° F, so is a problem in early spring (Younie et al., 2004) and survives a long time on pasture.
The climate, time of year, and species of parasite determine the management that will avoid the parasite. In the tropics, for example, Haemonchus larval levels peak one week after manure drop; levels drop so they are barely detectable on the pasture within four to six weeks (Waller, 2006). This is why, in the tropics, rotating every 3.5 days and then resting for 31.5 days lowered egg counts in goats to less than half the levels of set-stocked goats (“set-stocked” means they were left in place and not rotated). However, in cooler climates or during cooler seasons, the L3 (infective) larvae are slower to develop but are long-lived, surviving six to 18 months (O’Connor, 2007; Torres-Acosta and Hoste, 2008). In that situation, the same strategy that is successful in the tropics (returning to a pasture after 35 days) can be disastrous where animals are returning to a pasture when larvae are near the peak of infectivity. Some research has shown that rotational grazing increases the risk of internal parasite infection. On the other hand, Burke et al. tested a 3.5 day rotation, returning in 35 days for lambs grazing bermudagrass pastures in Arkansas. In that study, rotational grazing was effective in avoiding parasitism. The rotational group needed less deworming than those that were not moved (Burke et al., 2009c). Time of year must also be considered. In the Netherlands, researchers found that it took pastures three weeks to become highly infective with H. contortus in May and June, but only two weeks in July, August, and September. All important species of internal parasites in that environment decreased to low levels after about three months (Eysker et al., 2005). If animals are allowed to graze in infective areas (not rotated, or rotated back into an infective pasture too soon), they will consume larvae and repeat the cycle, thus multiplying contamination on the pasture.

This goat shows signs of severe parasite infection. Photo: J.M. Luginbuhl, NCSU
During development, larvae are vulnerable to prolonged drought and to cold and may also be destroyed by soil organisms, including earthworms (d’Alexis et al., 2009) and dung beetles (Stromberg, 1997). Internal parasites are usually specific to a species of host; that is, a sheep- or goat-parasite larvae will not readily develop inside cattle or horses, and vice versa. However, sheep and goats do share parasites. Some sheep have resistance to internal parasites, and those animals’ immune systems are better able to prevent larvae from establishing. Larvae consumed by an alternate livestock species or a resistant animal are “cleaned” from the pasture. Dry heat will also reduce pasture larval levels because the larvae need moisture to survive. Winter usually does not kill larvae on the pasture, so they will be waiting for spring warmth to hatch and become infective (Uriarte et al., 2003). Also, internal parasites have the ability to go into a kind of hibernation inside the animal; this is called “hypobiosis” and is a mechanism to help the parasite survive during times of the year that are not favorable to them outside the animal.
Weather conditions, the immune status of the animal, and pasture management techniques can all affect larval development and transmission. With time, larvae will naturally die if they are not ingested. However, pastures may have to rest a very long time to allow this natural cleaning: third-stage (infective) larvae (L3) can survive for one to three months in tropical or subtropical areas, but in temperate zones they may survive for six months to a year or more (Torres-Acosta and Hoste 2008). One of the keys to managing internal parasites is to understand the factors that suppress or encourage larval transmission. Here is a summary of those factors.
Internal parasites increase with:
• Warm, wet weather
• Hosts with low resistance
• Numbers of hosts
• Long periods of the same grazing animals on the pasture, so there are repeated cycles of ingestion and maturity and release of more eggs
Internal parasites are vulnerable to:
• Dry heat
• Non-host and resistant animals
• Time (enough time to die a natural death)
• Effective dewormers, including bioactive forages
• Soil organisms, including earthworms, nemaphatogous fungi, and dung beetles
Grazing Animals
Factors:
Class of animal
Stage of production
Quality and quantity of nutrition
Immune status
Numbers of animals
Larval intake
Type of forage consumed
Parasites impact grazing animals, but those animals may also affect the parasites. Sheep may develop the ability to resist parasites; that is, to stop the parasites from establishing inside the body, or to hinder the parasites from laying eggs. Goats seem to have less potential for resistance. It is thought that the grazing habits of sheep (a preference for short, tender forage) expose them to more internal parasite larvae, and the immune system then is stimulated to help the sheep inhibit the larvae. Goats have a different strategy for avoiding infection: a preference for browse (brush, vines, trees) and for wandering great distances, thus leaving areas of contamination (Hoste et al., 2010). Within groups of sheep or goats, there is variation in the ability of an individual animal to resist parasites. This is a heritable trait and managers are encouraged to select animals with resistance, because that is the best long-term solution for the internal parasite problem. Resistant animals suffer less parasitism and shed fewer parasite eggs, therefore reducing contamination on the pasture. Reduced contamination means less risk of parasitism for all animals. See the ATTRA publication Tools for Managing Internal Parasites in Small Ruminants: Animal Selection for information on identifying and selecting the most resistant animals.
Besides using resistant sheep or goats to lessen contamination on a farm, it is helpful to alternate cattle or horses with the sheep or goats. This works because the internal parasites are species specific. Sheep and goat parasites are removed by cattle grazing; cattle ingest the larvae, but the parasites do not readily establish and therefore do not multiply. Sheep and goats, however, do share parasites (as do llamas and alpacas). Many studies have been conducted that show the favorable results of alternating cattle grazing with sheep (Barger, 1996; Rocha et al., 2008; Thamsborg et al., 1999; Moss and Burton, 1998; Niezen et al., 1996). There is one caution: young calves may be infected with Haemonchus contortus. It is better to use adult cattle as alternate grazers in order to avoid this problem (Rocha et al., 2008).

Resistant animals, such as this Gulf Coast ewe, suffer less parasitism and shed fewer parasite eggs. Selecting resistant animals is the best long-term strategy for improving animal health. Photo: Linda Coffey, NCAT
Some animals are inherently more resistant than others. However, any animal will be less resistant at some stages in its production cycle. Susceptible animals are young lambs and kids and the ewes and does that are within a few weeks of giving birth or are nursing young. Pregnant and nursing females with twins or triplets are at greater risk than those with singles, due to the greater demand for protein and energy. In addition to the greater metabolic demands, those carrying twins or triplets have less room in their abdomen due to crowding from the fetuses, and they may not consume enough feed. After they are born, twins and triplets are also at greater risk. Because their mothers have less resistance, those mothers will deposit more eggs, increasing contamination on the pastures they share. Also, the twin and triplet lambs and kids will have less milk available than a single would.
Young animals have no immunity to internal parasites. This immunity develops slowly and only with exposure to internal parasites. Lambs acquire immunity at four to nine months of age, depending on the species of parasite and on exposure levels (Younie et al., 2004) and breed of sheep. This acquired immunity was seen in a study where lambs were infected with parasites, with peak egg counts seen when lambs were 11 weeks old. Six weeks later those counts had dropped three-fold, showing that lambs were expressing resistance (Athanasiadou et al., 2006). Ewes and lambs had a sudden drop in fecal egg count in August in Spain after showing signs of clinical disease (Uriarte et al., 2003). Organically raised lambs on another study were lagging behind conventionally raised lambs in their first year. The following year the trend was reversed, with the organic yearlings expressing resistance and gaining better than the conventional yearlings that were treated with anthelmintics (Niezen et al., 1996). These studies all demonstrate that animals have the ability to respond to internal parasite infection after exposure.
After immunity has developed, it may still be suppressed during times of stress (Vlassoff et al., 2001). This includes the time near the birth of young (called the “periparturient rise”) and during lactation, during illness, and whenever animal demands are greater than available nutrition. The extra need for nutrients explains why ewes and does nursing twins or triplets are more affected by parasites than those nursing singles (Kahn et al., 2003). Fecal egg counts (FECs) tend to be higher in ewes/does with low body condition score during mid-pregnancy, in yearlings as compared to older ewes/does, and for multiple-rearing compared to single-rearing ewes/does. Therefore, feeding these groups separately and providing supplementation to animals that need it will both be beneficial in reducing parasite infection in those animals and parasite contamination on the pastures.

This young doe is still growing and is feeding twins. She is at risk for increased parasite infection because of these stresses. Photo: Linda Coffey, NCAT
The body uses protein to rebuild tissues that are damaged by internal parasites, and supplementing animals with protein has been shown to improve immune response and overall health (Hoste et al., 2005; Kahn et al., 2003). How much protein will be needed? That depends on the forage base and the animals being fed. In one study, Merino ewes were supplemented with 250 g of cottonseed meal per day (about ½ pound) for either six weeks before birth of lambs or six weeks after, which resulted in a 66% reduction in fecal egg count (FEC) in both cases (Kahn et al., 2003). Merino lambs (five months old) in another study were supplemented with 100 g of cottonseed meal (31% crude protein) per head, per day, but were fed just twice weekly, at 350 g per feeding, to lower labor cost. The supplemented lambs had 44% higher gains than lambs without supplement, and 35% lower FEC (Eady et al., 2003). In Missouri, young lambs that were fed ¼ pound of soybean meal per day had higher gains and higher hematocrits (showing less anemia, expressing greater resistance to H. contortus) (Ross, 1989). Some experimentation will be necessary in your situation. In addition to protein, consider minerals, especially copper and zinc, which are associated with the immune system (Sykes and Coop, 2001). Also, when energy is limiting, an energy supplement is helpful (Valderrabano et al., 2002; Hoste et al., 2005). Supplementation of protein and energy can be provided through better-quality pasture, and this may be more economical than purchased supplements. Planting more legumes on the farm will improve soil and will improve the nutrition available to the animals. See ATTRA’s Ruminant Nutrition for Graziers for more information and consult your local Cooperative Extension Service to learn about legumes and other forages that may do well in your area.
While protein and other supplements are expensive, so is internal parasitism; animals that are parasitized will eat less. The problem accelerates as feed intake declines and the available nutrients are less as the needs are greater. Lactating animals will produce less milk while parasitized, so their lambs or kids will take in less nutrition and themselves be more likely to suffer parasitism and low growth rates. Also, parasitized animals shed more parasite eggs, contaminating the pastures for the rest of the grazing season. Animals that are losing weight due to poor forages or high nutritional demands will be more vulnerable to internal parasites. To boost immunity to parasites, managers can:
• Protect young animals from heavily contaminated areas
• Provide excellent nutrition to young, growing animals and to females just before parturition and during lactation
• Separate females nursing twins and triplets and offer extra feed
• Use low-stress handling techniques because stress lowers immunity

Goats browsing. Photo: Linda Coffey, NCAT
In addition to boosting immune systems, managers can protect their animals from parasites by offering access to legumes to provide more protein, to browse (Hoste et al., 2005), and to bioactive forages-that is, those with medicinal qualities, including chicory (Kidane et al., 2010) and sericea lespedeza (discussed in the next section). Giving access to plenty of available forage so that animals are not forced to graze close to the ground, where most larvae are usually found, will reduce intake of larvae and improve nutrition and intake of forage, helping the animals’ immunity. Having plenty of forage results in lower fecal egg counts (meaning less pasture contamination for the future) and animals in better health (Gazda et al., 2009).
Plenty of available forage is the result of adequate rainfall and an appropriate stocking rate. Dr. D.G. Pugh has stated that the correct stocking rate for sheep and goats is the point where you can grow all the forage needed for the year on the farm; that is, enough acreage that you could grow all the hay and grasses and browse needed for the animals (2003). This rule of thumb takes into account the soil productivity, normal rainfall, and forage types available on your farm. Drought years mean that managers need to respond by lowering animal numbers. The stocking rate affects the amount of available forage and also the numbers of internal parasite larvae being spread on the farm in manure.
Even with a reasonable stocking rate, a farm can be overgrazed and over-contaminated. This happens near water tanks, in shady areas, and near barns or favorite rest spots. Sometimes those areas can be fenced off, waterers moved, or other measures taken to rest overgrazed areas and allow larvae to die off. In addition to areas of heavy use, watch for wet areas: parasites thrive with moisture, so leaky troughs, faulty valves, and marshy areas will provide favorable microclimates for internal parasites. Take action to fix those problems or change the patterns of livestock movement when possible.
Animals can tolerate some numbers of internal parasite larvae, and larvae in small numbers are helpful in stimulating immunity against worms. Some animals that are infected at a young age exhibit greater resistance or tolerance to parasites as they get older (Niezen et al., 1996). The problem comes when numbers of parasite larvae overwhelm the immune system. To prevent illness, managers can work on two fronts: reduce exposure to parasite larvae and provide support for the animal’s immune system.
Tips for Animal Management
• Well-fed, healthy animals are better able to handle a parasite burden.
• Stressed animals tend to have reduced immunity and a poor ability to cope with worm infections.
• Young animals will not have immunity; it develops with time and exposure and may not be developed until four to nine months of age in lambs
• Animals that are not susceptible to internal parasites can clean a pasture for others; resistant animals, cattle or horses, and mature dry ewes are useful for this purpose.
Strategies to reduce exposure:
• Provide plenty of available forage
• Reduce stocking rate to appropriate levels
• Rest contaminated areas
• Give access to browse and bioactive forages
• Use resistant animals and alternate grazers (cattle, horses)
• Provide clean pastures for young and other susceptible stock
• Graze animals on regrowth from silage or hay crops
• Use annual forage crops, such as rye, turnips, or chicory (cool season) and sunn hemp, cowpeas, sorghum, or soybeans (warm season)
• Rotate animals away from larvae before they are infective
Strategies to provide support:
• Provide excellent nutrition (energy, protein, and minerals) to susceptible classes and during stressful times
• Allow limited exposure to parasite larvae to maintain immune response
• Provide diverse forages (browse, bioactive forages such as sericea lespedeza, a variety of plants) to encourage intake and give some medicinal benefits
Pastures
Factors:
Prior grazing (larval contamination)
Forage type
Secondary compounds, such as condensed tannins and others
Intensity of grazing
Length of rest
Species of livestock grazing (cattle, sheep or goats, horses)
Susceptibility of grazing animals
Weather
Pastures provide the environment for the eggs and the larvae. Knowing how to “clean” the pastures for susceptible animals will result in less worm infection and a more sustainable operation. To review the parasite life cycle, eggs hatch when moisture and temperature are favorable. During a hot, dry spell, many eggs and developing larvae will be destroyed by the heat and sunlight. Tilling the soil buries some eggs and larvae and exposes others to heat and light. Mowing or grazing close to the ground in hot weather can be helpful in exposing the eggs and larvae as well. Allowing the pastures a long rest from sheep and goat grazing will let the parasites die on the pasture without infecting animals. Outwaiting viable parasite larvae takes less time in a hot, dry summer, but takes months during cooler weather.
Some types of forage are especially helpful in reducing parasite problems in sheep and goats. Browse (brushy plants) will not have infective larvae on the leaves because larvae have difficulty migrating on that type of plant: the leaves are far enough from the manure to keep them “clean.” Browse may also have some medicinal properties; parasitized goats had lower fecal egg counts after being placed on browse without any other treatment (Hoste et al., 2005). Similarly, sericea lespedeza has been shown to have deworming properties in lambs (Burke et al., 2012a, b) when grazed. The researchers noted a shift during the study from H. contortus as the primary parasite to other species. In goat kids, where Trichostrongylus spp., not H. contortus, was the main parasite, sericea was not effective; however, goats were healthy and gained well (Burke et al., 2012b). For more about the use of sericea lespedeza in parasite control, see ATTRA’s Tools for Managing Internal Parasites in Small Ruminants: Sericea Lespedeza.
What are clean pastures?
Clean pastures are those with minimal risk of infection because the contamination of infective larvae is nil or very low when animals are introduced on the pasture. Clean pastures can be obtained through new reseeds, silage aftermaths, or annual forage crops. Pastures that have not been grazed by stock of the same species within the year can also be considered clean (Younie et al., 2004).WARNING:
For safe ways to use dewormers so that resistance is minimized, see the ACSRPC website. Do not chemically deworm animals and move to clean pasture-this encourages development of dewormer resistance.
The cleanest and most nutritious pastures should be offered to the most susceptible animals (young lambs and kids, females nursing multiple lambs and kids) because these are the animals that are most vulnerable to illness and are shedding the most eggs to contaminate pastures for the rest of the season. To protect those animals and lower the risk of parasitism:
• Supply safe grazing with newly established pastures or crop aftermath or pastures not grazed by sheep or goats for a year
• Provide ample quantities of nutritious forages
• Offer supplements (protein, energy, minerals) to boost immunity
• Plant bioactive (medicinal) forages such as chicory, sulla, birdsfoot trefoil, panicle tick clover, Kobe lespedeza, and sericea lespedeza
• Offer browse
• Do not allow animals to graze pastures too short
• Let the susceptible classes graze first, or let them follow resistant animals to lower intake of larvae
Pasture Management

Sericea lespedeza is readily consumed by goats and sheep and will help control internal parasites. Photo: J.M. Luginbuhl, NCSU
We have discussed the internal parasite life cycle and the factors that drive it on the pasture, the animal production cycle and individual resistance and how they affect the pasture contamination level, and the aspects of the pasture that influence contamination and nutrition. We have presented techniques that protect animal health and strategies to lower the contamination level of pastures. However, there are no formulas or recipes that will keep you from having issues with internal parasites on your farm.
Tools to help integrate the multiple management concepts listed above are being created. These decision trees may be available online in the future and will allow producers to get the help of computers in sorting through the complexities of pasture, animal, and parasite interactions. In the meantime, managers have to develop the habit of thinking about all three aspects (parasites, animals, and pastures) of the farm at once. Keeping grazing records so that you know when you left a pasture is important. Having a plan that allows a long rest period while also maintaining good forage quality and quantity for animal health and nutrition will be useful. Remember to note in the plan which animals are grazing first (those most susceptible) and to send the animals with highest nutritional requirements to your best, most nutritious pastures.

Grazing turnips in the fall provides sheep and goats with “clean” grazing and excellent nutrition during breeding season. Photo: Linda Coffey, NCAT
Rotating pastures is key to preventing internal parasitism. Keeping animals on the same pasture for multiple parasite life cycles will greatly increase contamination on the pasture and parasite levels in the animals, increasing the risk of illness. Under optimum conditions, Haemonchus contortus completes a life cycle in 21 to 25 days. However, animals that already have mature worms will be shedding eggs on Day 1, and those eggs can hatch and have infective larvae by Day 4 or 5. This is the rationale for moving just before Day 4 (Burke et al., 2009c). Langston University research showed that moving goats after five days was adequate to escape parasitism over the summer (Pomroy et al., 2002).
Short grazing times (four to five days) during warm, moist weather would then seem to make sense to avoid picking up newly infective parasite larvae. When is it safe to re-graze a pasture? Unfortunately, that is a difficult question. The answer will depend on what species of internal parasite(s) are present, the temperature and moisture conditions, immune status of the grazing animals, and perhaps the type of forage (e.g., density of the stand may impact larvae survival). It takes a very long time for pastures to self-clean. Most farms do not have enough land to allow a pasture rest period that will ensure that their grazing animals are perfectly safe from parasites. The larvae can survive for months, although in hot weather they will not live as long. In Oklahoma, at Langston University, researchers had good results from resting pastures 60 days (Haemonchus contortus is prevalent in that region). Using multispecies grazing or resistant animals to consume the infective larvae, then letting the pasture re-grow before coming back with sheep or goats is a good protective strategy. Cutting for hay will also help because it removes some larvae and exposes others to heat and sunlight.

This sheep is getting no nourishment but plenty of parasites in this situation. Photo: Linda Coffey, NCAT
Maintaining adequate forage height is important for avoiding parasite infection and providing good nutrition to the animals, as well as allowing pastures to maintain health and grow back quickly. However, putting this concept into practice can be a challenge. Sheep especially have a tendency to spot graze. They will leave taller forage and continue to graze much shorter, new-growth forage. This means they are grazing areas very close to the ground. Close observation of forage height is important; move animals to a new pasture before forage height is below four inches. This will help grass recovery as well as limit intake of infective larvae. Again, following with a more resistant class (such as dry ewes or cattle) may allow somewhat shorter grazing, and this will expose larvae to sunlight and reduce their numbers. However, grazing too short will impact plant survival and regrowth.
If your pastures always seem “too short” and you aren’t able to give them enough rest time before moving animals back onto them, you are likely overstocked. Reducing animal numbers will help alleviate overgrazing. It is best if you sell those animals that have the most problems with worms to reduce pasture contamination and stocking rate at the same time. If you can gain access to more (and fresh) pastures by renting a neighbor’s land, that will be a great help in evening out forage supply and demand and giving your home pastures a rest. If that is not possible, you may have to feed hay (particularly during a drought) or give other supplementary feed. Steadily monitoring the condition of the pastures and animals and regularly reviewing your grazing plan are critical. Because pasture growth depends on rainfall, it will be different every year, calling for corresponding adjustment of management strategies.
Principles of pasture management for animal health
• Maintain proper forage height
• Maintain some “clean grazing” areas
• Manage problem areas
• Maintain proper stocking rate
• Use multispecies grazing
• Use leader-follower grazing (lead with susceptible classes, follow with less susceptible; for example, lead with lambs and follow with cattle or dry ewes)
• Offer diverse forages and browse
• Use rotational grazing with long rest periods
If your farm situation allows, setting aside a different part of the farm for replanting each year can be a big help in providing clean grazing for susceptible animals, and in offering the chance to establish permanent pastures that include areas of medicinal forages and legumes to increase protein. Giving access to browse areas is helpful, though browse requires very long rest periods. There are difficulties in replanting: these include cost, risk of erosion, establishment time, and labor and time. Not every farm lends itself to tillage or to idling land for replanting. If totally reseeding a pasture isn’t an option, consider overseeding legumes. Doing what you can to improve organic matter and soil fertility will help pastures be as productive as possible.
Pasture management is challenging. Keeping records (grazing plan, animal numbers, rainfall amounts, parasite treatments needed) will help you fine-tune a plan that works for your farm.
Tools for Managing Parasites
In addition to pasture management, there are many tools for managing internal parasites. Due to the complex nature of parasite control, it is necessary to use multiple management techniques to combat the problem. The following are some tools that can be used to manage internal parasites. Using more of the tools will improve results.
- Animal management (discussed in this publication and in the ATTRA publication Managing Internal Parasites in Sheep and Goats)
- Selective deworming and FAMACHA©. See Managing Internal Parasites in Sheep and Goats.
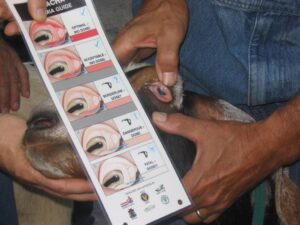
Use FAMACHA© to assess levels of anemia. Photo: Margo Hale, NCAT
- Use FAMACHA for classifying animals based on levels of anemia (according to eye mucous-membrane color).
- Treat only animals with symptoms of anemia.
- Deworm selectively to reduce use of dewormers, which slows development of resistance and saves money.
- Remember FAMACHA is only effective for the treatment of H. contortus.
- Use the Five-Point Check to pick up signs of other parasites. Again, only treat animals with symptoms, not the whole flock or herd. See ATTRA’s Tools for Managing Internal Parasites in Small Ruminants: Animal Selection.
- Keep records to show which animals are more resistant or resilient, and retain those animals for breeding.
- Selecting resistant animals
- Alternative control methods
- Copper oxide wire particles have been found to reduce parasite loads in sheep and goats. See Tools for Managing Internal Parasites in Small Ruminants: Copper Wire Particles.
- Forages with high levels of condensed tannins, such as Sericea lespedeza, have been shown to reduce parasite loads. See Tools for Managing Internal Parasites in Small Ruminants: Sericea Lespedeza.
- There are anecdotal claims that botanicals such as garlic, papaya seeds, pumpkin seeds, and herbal dewormers are effective means of parasite control. However, controlled research on these methods has shown they have no effect on parasites (O’Brien et al., 2012; Burke et al., 2009a,b).
Use the attached assessment sheets to help you integrate as many tools as possible as you manage your pastures, animals, and parasites.
Summary
Pasture management is a fundamental tool in controlling internal parasites, and none of the other tools will be effective without good pasture management. Therefore, spending time and attention (and money) on doing a good job is well worth the investment. Managing pasture and animals to provide adequate nutrition for each stage of production and to avoid contact with infective internal parasite larvae will result in improved health and production for grazing animals. Pasture management is a vital component of a holistic parasite-management strategy.



References
Amaradosa, B.S., R.A. Lane, and A. Manage. 2010. Veterinary Parasitology. Vol. 10. p 78-87.
Athanasiadou, S., D. Gray, D. Younie, O. Tzamaloukas, F. Jackson, and I. Kyriazakis. 2006. The use of chicory for parasite control in organic ewes and their lambs. Parasitology. Vol. 134. p 299-307.
Barger, I. A. 1996. Prospects for integration of novel parasite control options into grazing systems. International Journal for Parasitology. Vol. 26: No. 8-9. p. 1001-1007.
Burke, J.M., A. Wells, P. Casey, and J.E. Miller. 2009a. Garlic and papaya lack control over gastrointestinal nematodes in goats and lambs. Veterinary Parasitology. Vol. 159. p. 171-174.
Burke, J.M., A. Wells, P. Casey, and R.M. Kaplan. 2009b. Herbal dewormer fails to control gastrointestinal nematodes in goats. Veterinary Parasitology. Vol. 160. p. 168-170.
Burke, J.M., J.E. Miller, and T.H. Terrill. 2009c. Impact of rotational grazing on management of gastrointestinal nematodes in weaned lambs. Veterinary Parasitology. Vol. 10. p. 1016-1021.
Burke, J.M., J.E. Miller, J.A. Mosjidis, and T.H. Terrill. 2012a. Grazing sericea lespedeza for control of gastrointestinal nematodes in lambs. Veterinary Parasitology. Vol. 186. p 507-512.
Burke, J.M, J.E. Miller, J.A. Mosjidis, and T.H. Terrill. 2012b. Use of a mixed sericea lespedeza and grass pasture system for control of gastrointestinal nematodes in lambs and kids. Veterinary Parasitology. Vol. 186. p. 328-336.
D’Alexis, S., G. Loranger-Merceris, M. Mahieu, and M. Boval. 2009. Influence of earthworms on development of the free-living stages of gastrointestinal nematodes in goat faeces. Veterinary Parasitology. Vol. 163. p. 171-174.
Eady, S.J., R.R. Woolaston, and I.A. Barger. 2003. Comparison of genetic and nongenetic strategies for control of gastrointestinal nematodes of sheep. Livestock Production Science. Vol. 81. p. 11-23.
Eysker, M., N. Bakker, F.N.J. Kooyman, and H.W. Ploeger. 2005. The possibilities and limitations of evasive grazing as a control measure for parasitic gastroenteritis in small ruminants in temperate climates. Veterinary Parasitology. Vol. 129. p. 95-104.
Gazda, T.L., R.G. Piazzetta, J.R. Dittrich, A.L.G. Monteiro, and V. Thomaz-Soccol. 2009. Distribution of nematode larvae of sheep in tropical pasture plants. Small Ruminant Research. Vol 82. p. 94-98.
Gordon, H. McL. 1967. Diagnosis of helminthosis in sheep. Veterinary Medical Review. Vol. 2/3. p. 140-168.
Hoste, H., S. Sotiraki, S.Y. Landau, F. Jackson, and I. Beveridge. 2010. Goat-Nematode interactions: think differently. Trends in Parasitology. Vol. 26. p. 275-281.
Hoste, H., J.F. Torres-Acosta, V. Paolini, A. Aguilar-Caballero, E. Etter, Y. Lefrileux, C. Chartier, and C. Broqua. 2005. Interactions between nutrition and gastrointestinal infections with parasitic nematodes in goats. Small Ruminant Research. Vol. 60. p. 141-151.
Hutchens, Terry and Monty Chappell. 2004. Gastro-Intestinal Parasite Survival Kit for Goats
Kahn, L.P., M.R. Knox, S.W. Walkden-Brown, and J.M. Lea. 2003. Regulation of the resistance to nematode parasites of single- and twin-bearing Merino ewes through nutrition and genetic selection. Veterinary Parasitology. Vol. 114. p. 15-31.
Kidane, A., J.G.M. Houdijk, S. Athanasiadou, B.J. Tolkamp, and I. Kyriazakis. 2010. Effects of maternal protein nutrition and subsequent grazing on chicory (Cichorium intybus) on parasitism and performance of lambs. Journal of Animal Science. Vol 88. p. 1513-1521.
Leathwick, D.M., C.M. Miller, and T.S. Waghorn. 2011. Development and spatial distribution of the free-living stages of Teladorsagia circumcincta and Trichostronglylus colubriformis on pasture: A pilot study. New Zealand Veterinary Journal. Vol. 59, No.6. p.272-278.
Moss, R.A., and R. N. Burton. 1998. Effect of cattle grazing strategies and pasture species on internal parasites of sheep. New Zealand Journal of Agricultural Research. Vol. 41. p. 533-544.
Niezen, J.H., W.A.G. Charleston, J. Hodgson, A.D. Mackay, and D.M. Leathwick. 1996. Controlling internal parasites in grazing ruminants without recourse to anthelmintics: Approaches, experiences and prospects. International Journal for Parasitology. Vol. 26. p. 983-992.
O’Brien, D.J, N.C. Whitley, J.E. Miller, J.M. Burke, K.K. Matthews, and M.C. Gooden. 2012. Efficacy of pumpkin seeds and ginger in controlling gastrointestinal nematodes (GIN) in meat goat kids. Unpublished paper.
O’Connor, L.J., L. P. Kahn, and S. W. Walkden-Brown. 2007. Moisture requirements for the free-living development of Haemonchus contortus: Quantitative and temporal effects under conditions of low evaporation. Veterinary Parasitology. Vol. 150. p. 128-138.
Pomroy, W. E., S. P. Hart and B. R. Min. 2002. Rotational grazing as a parasite management tool for goats. Journal of Animal Science. Vol. 80, Supplement 1. p. 193.
Pugh, D. G. Nashville, TN. 2003. Presentation. American Dairy Goat Association Convention
Rocha, R.A., K.D.S. Bresciani, T.F.M. Barros, L.H. Fernandes, M.B. Silva, and A.F.T. Amarante. 2008. Sheep and cattle grazing alternately: Nematode parasitism and pasture decontamination. Small Ruminant Research. Vol. 75. p. 135-143.
Ross, C.V. 1989. Sheep Production and Management. Prentice-Hall, Inc. Englewood Cliffs, New Jersey. p. 206.
Santos, M.C., B.F. Silva, and A.F.T. Amarante. 2012. Environmental factors influencing the transmission of Haemonchus contortus. Veterinary Parasitology. Doi:10.1016/j.vetpar.2012.03.056. (accepted manuscript, unedited)
Silva, B.F., M.R.V. Amarante, S.M. Kadri, J.R. Carrijo-Mauad, and A.F.T. Amarante. 2008. Vertical migration of Haemonchus contortus third stage larvae on Brachiaria decumbens grass. Veterinary Parasitology. Vol. 158. p. 85-92.
Stromberg, B.E. 1997. Environmental factors influencing transmission. Veterinary Parasitology. Vol. 72. p. 247-264.
Sykes, A.R., and R.L.Coop. 2001. Interaction between nutrition and gastrointestinal parasitism in sheep. New Zealand Veterinary Journal. Vol. 49, No. 6. p. 222-226.
Thamsborg, S.M., A. Roepstorff, and M. Larsen. 1999. Integrated and biological control of parasites in organic and conventional production systems. Veterinary Parasitology. Vol. 84. p. 169-186.
Torres-Acosta, J.F.J., and H. Hoste. 2008. Alternative or improved methods to limit gastro-intestinal parasitism in grazing sheep and goats. Small Ruminant Research. Vol. 77. p. 159-173.
Uriarte, J., M.M. Llorente, and J. Valderrabano. 2003. Seasonal changes of gastrointestinal nematode burden in sheep under an extensive grazing system.Veterinary Parasitology. Vol. 118. p. 79-92.
Valderrabano, J., R. Delfa, and J. Uriarte. 2002. Effect of level of feed intake on the development of gastrointestinal parasitism in growing lambs. Veterinary Parasitology. Vol. 104, No.4. p. 327-338.
Vlassoff, A., D.M. Leathwick, and A.C. Heath. 2001. The epidemiology of nematode infections of sheep. New Zealand Veterinary Journal. Vol. 49, No. 6. p. 213-221.
Waller, P.J. 2006. Sustainable nematode parasite control strategies for ruminant livestock by grazing management and biological control. Animal Feed Science and Technology. Vol. 126. p. 277-289.
Younie, D., S. Thamsborg, F. Ambrosini, and S. Roderick. 2004. Grassland Management and Parasite Control. In: M. Vaarst, S. Roderick, V. Lund and W. Lockeretz (eds.). Animal Health and Welfare in Organic Agriculture. CABI, Wallingford Oxfordshire.
Further Resources
American Consortium for Small Ruminant Parasite Control (ACSRPC)
ACSRPC was formerly known as the Southern Consortium for Small Ruminant Parasite Control (SCSRPC) and provides up-to-date scientific research and recommendations for producers. There are many helpful articles listed on the site, including information about FAMACHA© and Smart Drenching.
Sustainable Agriculture Research and Education (SARE)
The SARE website has many research reports of interest to sheep and goat producers. To access these reports, go to the homepage, click on “project reports” and then search “internal parasite” to bring up a list of reports that can be informative on this subject. As of this writing, there are 76 projects related to this topic, with many about pasture management and alternative forages.
Langston University
Langston University’s web-based training manual
See especially:
Chapter 7 (Internal and External Parasite Management),
Chapter 10 (Introduction to Goat Nutrition), and
Chapter 11 (Pastures for Goats)
Maryland Small Ruminant Page
Susan Schoenian is an educator with the University of Maryland Cooperative Extension Service. She has generously shared information with the world through this website. She also has posted some excellent presentations at Slideshare, including some about integrated parasite management. These presentations are very helpful and will add to understanding of the problem and solutions. Access them from the main website.
Ohio State University-Sheep Team Parasite Resources
Parasite Management Presentations
Strategies for Coping with Parasite Larvae on Pastures in the Springtime in Ohio
Tools for Managing Internal Parasites in Small Ruminants: Pasture Management
By Linda Coffey and Margo Hale, NCAT Sustainable Agriculture Specialists
Published October 2012
©NCAT
IP401
Slot 395
This publication is produced by the National Center for Appropriate Technology through the ATTRA Sustainable Agriculture program, under a cooperative agreement with USDA Rural Development. This publication was also made possible in part by funding from the USDA, NIFA, OREI, award 2010-51300-21641; and the Southern Region Sustainable Agriculture Research and Education, award 2005-51300-02392. ATTRA.NCAT.ORG.
Related Publications
- Managing Internal Parasites in Sheep and Goats
- Pasture, Rangeland, and Adaptive Grazing
- Ruminant Nutrition for Graziers
- Small Ruminant Sustainability Checksheet
- Tools for Managing Internal Parasites in Small Ruminants: Animal Selection
- Tools for Managing Internal Parasites in Small Ruminants: Copper Wire Particles
- Tools for Managing Internal Parasites in Small Ruminants: Sericea Lespedeza


