Symphylans: Soil Pest Management Options
By Jon Umble, Oregon State University; Rex Dufour, NCAT; Glenn Fisher, Oregon State University; James Fisher, USDA/ARS; Jim Leap, University of California, Santa Cruz; Mark Van Horn, University of California, Davis

A garden symphylan is about the size of this letter ”l.”
Abstract
Garden symphylans are soil-dwelling, centipede-like creatures that feed on plant roots and can cause extensive crop damage. They cause frequent and often misdiagnosed problems in well managed western soils with good tilth. This soil pest may not be familiar to farmers and agricultural consultants. This publication describes the garden symphylan life cycle and the damage symphylans can cause. It includes monitoring techniques to determine whether symphylans are present in the soil and sustainable management options to prevent economic damage.
Contents
Introduction
Damage
Identification
Biology and Ecology
Sampling
Management and Control
References
Introduction

Soils with high organic matter, good structure, and
reduced disturbance, as in these hand-dug garden beds, are ideal habitat for garden symphylans.
Garden symphylans (Scutigerella immaculata Newport) are small, white, centipede-like soil arthropods, common in many agricultural production systems in Oregon, Washington, and California (Berry and Robinson, 1974; Michelbacher, 1935).
They feed on roots and other subterranean plant parts, causing significant crop losses in some cases. Control can be extremely difficult due to symphylans’ vertical movement in the soil, the complexity of sampling, and the lack of simple, effective control methods (Umble and Fisher, 2003a).
With the recent spread of organic agriculture and better soil management techniques, crop damage associated with symphylans has become more commonplace. It is ironic that these pests are such a problem on farms that practice good soil management — maintaining soil with good tilth, high organic matter, and low compaction.
Damage
Diagnosing a garden symphylan problem is sometimes difficult, since damage may be exhibited in a number of forms and garden symphylans are not always easy to find, even when their damage is obvious. Economic damage may result from direct feeding on root and tuber crops and reduced stands of direct seeded or transplanted crops (Umble and Fisher, 2003a).
However, most commonly, root feeding reduces the crop’s ability to take up water and nutrients, which leads to general stunting. Root damage may also render plants more susceptible to some soil-borne plant pathogens. Correct diagnosis of
garden symphylan problems and identification of appropriate management tactics for a given cropping system will generally require the following:
- Sampling to determine whether garden symphylans are present in damaging numbers
- A general knowledge of management tactics and garden symphylan ecology to select the specific tactics that will be most effective in a given cropping system

Eggplant stunted by garden symphylans.

Undamaged eggplant of same age in same field.

Symphylan damage can be hard to diagnose because it may seem to be from other causes. This photo show two rows of eggplant, one that is severely stunted and one less so. Neither the rows to the left nor the peppers on the right seem to be affected. It is likely that the timing of planting and tillage influences the amount of damage that symphylans cause. Many factors may interact to promote or reduce symphylan damage: conditions when the soil is tilled, time after tillage, heat requirements of the crop, irrigation management, size of the plant, etc.


Typical perplexing symphylan damage: Some rows of peppers (above, top) are severely stunted, while adjacent rows have both healthy and stunted plants. Symphylan damage can be mistaken for skipped seeding or poor seed-to-soil contact, as in these fields of squash (above, bottom), sweet corn, and tomatoes (below)



Certain areas of this squash field are laid to waste by symphylans, while other sections thrive.

Typical symphylan damage, showing healthy plants alongside stunted plants and empty areas.
Identification
Garden symphylans are not insects, but members of the class Symphyla. Species of this class are common soil arthropods worldwide. Symphyla are small, whitish centipede-like creatures ranging from less than 1/8 inch up to about 5/8 inch (or 1/4 inch for garden symphylans) (Edwards, 1990). They have six to 12 pairs of legs (depending on age), which makes them easy to distinguish from common soil insects (e.g., springtails) and diplurans that have only three pairs of legs, all on the thorax, or middle body segment.
Though their color may vary, depending on what they have eaten, garden symphylans are generally whiter and smaller than true centipedes, which are also soil arthropods with many pairs of legs (one pair per body segment) and quick movements. Millipedes are generally slower moving, with two pairs of legs on each body segment.
Some Symphyla species feed primarily on dead or decaying organic matter, playing an important role in cycling nutrients. Other species, such as the garden attsymphylan, are serious pests, primarily feeding on living plants.
Several Symphyla species are present in the western United States; however, the garden symphylan is the only Symphyla species that is documented to cause crop damage in the western U.S. Garden symphylans are by far the most common Symphyla species found in agricultural systems.
If Symphyla are found in an agricultural system at a high density, concentrated around the roots of plants, they are likely to be garden symphylans. The pest is not known to vector any plant diseases, although extensive research on the question has not been conducted.
Garden Symphylan Biology and Ecology
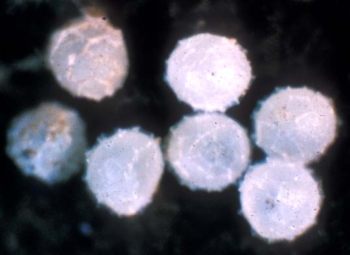
Garden symphylan eggs. Photo: Ralph Berry
Life Cycle
In the western U.S., eggs, adults, and immature garden symphylans can be found together throughout most of the year. Temperature plays a key role in regulating oviposition (egg laying), and the greatest numbers of eggs are usually deposited in the spring and fall (Berry, 1972).

Newly emerged symphylans (first instar). Photo credit: Ralph Berry
Eggs are pearly white and spherical with hexagonal shaped ridges. Eggs incubate for about 25 to 40 days, when temperatures range from 50° to 70°F, but hatching occurs in about 12 days as temperatures reach 77°F (Berry, 1972).
First instars emerge from the egg with six pairs of legs and six antennal segments, their bodies covered with fine hairs. Slow movements and a swollen posterior make first instars appear superficially more like a collembolan than an adult garden symphylan. These first instars, however, are rarely found in the rooting zone and within days molt to second instars that resemble small adult garden symphylans (Michelbacher, 1938).

Mature adult garden symphylan.
Each of the six subsequent molts results in the addition of a pair of legs and variable numbers of body and antennal segments. Total time from egg to sexually mature adult (seventh instar) is about five months at 50°F, decreasing to about three months at 70°F and less than two months at 77°F. Therefore, it may be possible to have two complete generations a year (Berry, 1972). Interestingly, unlike adult insects, which do not molt, adult garden symphylans may molt more than 40 times (Michelbacher, 1938).
Occurrence
Understanding of garden symphylan occurrence and movement is far from
complete. Nonetheless, some generalizations can be made both about soils in
which garden symphylans occur more commonly and about their movement in
soils. Garden symphylan populations are highly aggregated within fields and
on a larger scale.

Fields often show symphylan damage in the same places over many seasons, as on these two farms.

In Oregon, Washington, and California, garden symphylans are more common in the western regions of the states. Within these regions, garden symphylans tend to occur in heavier irrigated soils, and within these heavier soils, garden symphylans tend to occur in “hotspots” of a few square feet to several acres. Even within shovelfuls of soil, garden symphylans often occur in very distinct aggregations.
Garden symphylans are unable to burrow through the soil. They use pores, seasonal cracks and burrows made by other soil animals, such as earthworms, to travel through the soil profile (Edwards, 1961).

Pores, cracks, and holes in the soil allow symphylans
to move through a field with relative ease.
In general, practices that improve soil structure (e.g., addition of organic matter, reduced tillage, raised beds) improve the ability of garden symphylans to move through the soil, leading to increased populations and/or increased damage through improved access to roots. As a result, high populations of garden symphylans are more commonly found in fine-textured, heavier soils with moderate or better structure and many macropores, rather than in sandy soils (Edwards, 1958; Edwards, 1961). When garden symphylans are found in sandier soils, these soils have commonly been amended with organic matter.
In the Pacific Northwest and Northern California, garden symphylans are commonly found in alluvial soils, and are likely spread to some extent by flooding. Relative soil acidity does not appear to be closely correlated with garden symphylan occurrence, since symphylans are found in very acid soils (e.g., where blueberries grow) to fairly alkaline soils (e.g., pH 8+).

“Hotspots” of garden symphylan infestation show
clearly in this aerial view of a broccoli field.



Above: Three views of one broccoli field showing symphylan damage.
Hot spots within infested fields often remain consistent from year to year with little change in populations and only minor lateral spread, possibly due to physical characteristics of the soil. However, changes in hotspots do occur (Umble and Fisher, 2003c).
Symphylan Movement in the Soil and Factors Influencing Population Levels
If the soil environment is favorable, garden symphylans may migrate from the soil surface to a depth of more than 3 feet. The soil profile, including compacted or sandy horizons and high water tables that may impede movement, determines the depth to which garden symphylans may migrate. Timing of vertical migrations is primarily due to the interaction among moisture, temperature, and internally regulated feeding cycles (Edwards, 1959b). A general understanding of these interactions is important both for the timing and interpretation of sampling efforts and for selecting management tactics.
Garden symphylans tend to aggregate in the top six inches of soil when the soil is moist and warm, and move to deeper soil strata when soil becomes very dry or cool. In Oregon, Washington, and California, garden symphylans are generally found in the surface soil from March through November, with the highest surface populations observed in May and June. Garden symphylans may be found in the surface soil when conditions are fairly warm (e.g., when air temperatures exceed 95°F), if sufficient moisture is present and roots are shallow or absent. In the hottest interior valleys, symphylans may be more active in the spring/early summer and the fall, with surface activity dropping off in July, August, and into September.
Garden symphylans migrate to the surface soil (the root zone) to feed, then return to the deeper strata to molt, as demonstrated by the large number of molted skins that are observed in these strata. When garden symphylans are feeding ravenously after molting, they may enter the surface soil zone even in generally unfavorable (e.g., hot and dry) conditions. Since migrations are not synchronized, portions of the population are usually present throughout the habitable portion of the soil profile (Edwards, 1959b). Presence of garden symphylans in the surface soil may also be influenced by other variables that impede movement, such as tillage and compaction from tractor tires.
Sampling
Sampling for garden symphylans is extremely important for identifying damage, for making informed management decisions, and for evaluating the effects of those decisions. Sampling, however, is often difficult. Three main sampling methods are used: baiting, soil sampling, and indirect sampling. Each method has benefits and drawbacks, and the selection of a sampling method will vary, depending on the objectives of the sampling (e.g., detection vs. precise estimate of population density), the time of year, and the site conditions.
Part of the difficulty in sampling is due to the patchy distribution of populations. It is important to be aware that an individual sample count provides information only about the region near where that sample was taken. Counts will often vary from 0 to more than 50 garden symphylans per sample. To get information about the spatial patterns of the population, it is best to take sample units in a grid pattern. Sorting and comparing the samples by site factors such as soil type, drainage, and cropping history may provide valuable information about the distribution of populations within a site.
General Decision Guidelines for Sampling
To detect or identify a symphylan problem with a growing crop:
- Dig up stunted plants and weeds in the morning; examine their roots for symphylans.
- If it’s within about three weeks after planting, put out baits for garden symphylans in suspected problem areas.
To estimate population density and/or make decisions before planting a crop:
- Take soil samples if the soil is cool or very dry, if the field is very weedy, or if a cover crop is growing.
- Use bait sampling if the soil is warm and moist with sparse vegetation or if the soil is bare.
In most cases, sampling measures only the density of symphylans in the surface soil; therefore, sampling should only be conducted when garden symphylans are in the surface soil. The best sampling conditions are generally warm, moist soil. Sampling within three weeks after major tillage—such as discing, plowing, or spading—is often inaccurate, since garden symphylans may not have had ample time to re-establish in the surface soil. If sampling is conducted soon after tillage, soil sampling methods should be used. Sampling should be conducted to a depth that includes several inches of soil undisturbed by tillage.
Soil Sampling
Soil sampling is the standard/historic method for estimating how many garden symphylans are present in a field (i.e., approximate number of garden symphylans per unit of soil, or estimated population density) (Berry and Robinson, 1974). Sample unit sizes vary. The most common soil sample units have been the following:
- A 1-foot cube
- A 6-inch square, 1-foot deep
- A “shovelful”
- Cores 3 to 4 inches in diameter and 4 to 12 inches deep

Soil sampling in corn is carried out by placing soil on a black tarp and then carefully searching for garden symphylans.
When soil samples are taken, the soil from each sample unit is usually placed on a piece of dark plastic or cloth, where the aggregates are broken apart and the garden symphylans are counted (Berry and Robinson, 1974).
Sampling must be conducted throughout the entire habitable region of the soil profile (i.e., possibly to a depth of more than three feet) to obtain accurate population density estimates, but this is rarely done, because of the extensive time and resources required. Therefore, sampling is usually conducted when garden symphylans are believed to be in the top 6 to 12 inches of the root zone. Shallow sampling (e.g., to a depth of 4 inches) saves time and allows larger areas to be sampled, but deeper sampling (e.g., to a depth of 12 inches) is generally more reliable. Sampling is not recommended in very dry conditions.
Bait Sampling
 In recent years, bait sampling methods have been developed. Bait samples are generally much faster to take than soil samples, but they are also more variable and more sensitive to factors such as soil moisture, temperature, and the presence of vegetation (Umble and Fisher, 2003b). To bait sample, place half of a potato or beet on the soil surface and shelter it with a protective cover (e.g., a white pot or a 4-inch PVC cap).
In recent years, bait sampling methods have been developed. Bait samples are generally much faster to take than soil samples, but they are also more variable and more sensitive to factors such as soil moisture, temperature, and the presence of vegetation (Umble and Fisher, 2003b). To bait sample, place half of a potato or beet on the soil surface and shelter it with a protective cover (e.g., a white pot or a 4-inch PVC cap).
 One to three days after placement, lift the bait and count first the garden symphylans on the soil and second the garden symphylans on the bait. During warm and/or dry conditions, baits are generally checked one to two days after placement, as counts decrease if baits are left out for multiple days. In cooler conditions, baits are commonly left out for three to five days.
One to three days after placement, lift the bait and count first the garden symphylans on the soil and second the garden symphylans on the bait. During warm and/or dry conditions, baits are generally checked one to two days after placement, as counts decrease if baits are left out for multiple days. In cooler conditions, baits are commonly left out for three to five days.

Photos above: Using a cut potato as bait to check for the presence of garden symphylans.
Bait sampling works very well for some applications, though it cannot be used in all conditions. Baiting works best at least two to three weeks after tillage, when the soil has stabilized, but before plants are well established. If travel to the sampling location requires significant resources, soil sampling methods may be preferred, because they require only one trip to the site.
Indirect Sampling Methods
Plant growth can sometimes be a useful indirect measure of garden symphylan populations and is often a good starting point for assessing their spatial patterns (Umble and Fisher, 2003a). For example, healthy plants sometimes indicate low garden symphylan populations and conversely, unhealthy plants sometimes indicate high garden symphylan populations.
How Many Soil or Bat Sample Units to Use?
When sampling for garden symphylans, a critical number of sample units (i.e., “chunks” of soil or baits) are required in order to have a reasonable level of confidence about the estimated population density (e.g., garden symphylans per square foot) (Umble and Fisher, 2003b). Confidence in this estimate increases as more samples are taken. Sampling involves establishing a balance between wanting to be fairly confident about the number of garden symphylans present (taking a large number of samples) and not investing excessive time and energy in the sampling endeavor (taking a small number of samples).
Use the following general guidelines to determine the appropriate number of sample units.
- Simply detecting the presence of garden symphylans may only require digging up five to 10 damaged plants, or using a low number of baits (e.g., five)
- Sampling for low population densities (early in the spring or in highly susceptible crops) requires more sample units (e.g., 100+) than sampling for high population densities (e.g., 30)
- As the variability of a sampling method increases, so does the number of sample units required. Since the baiting method is more variable than the soil sampling method, two to three times more baits are required than soil samples
- To estimate “economically damaging” population densities in moderately susceptible crops, at least 35 soil sample units or at least 50 bait samples are commonly set out. Depending on the size of the field and the time of year, considerably more sample units may be used.
Indirect measures such as this may provide valuable information about the extent and pattern of infestation, but they should not be used in place of direct sampling. This is because many factors could lead to healthy plant growth, even within infested soil (e.g., the planting date, tillage intensity, chemical use, and crop species).


Spinach seedlings show susceptibility to symphylan damage. The soil in the pot in the upper photo contains 45 symphylans. Soil in the pot in the lower photo has no symphylans in it.
Action Thresholds: Interpretation of Sampling Results
Management decisions, such as those regarding pesticide applications and the intensity of tillage, are sometimes based on pre-planting density estimates of garden symphylan population. Owing partially to the many crops in which garden symphylans are pests, thresholds for individual crops are not well developed. The relationship between garden symphylan population densities (estimated by sampling methods) and measures such as stand count and yield are influenced by factors such as crop type, tillage intensity, and crop stage (Umble and Fisher, 2003a).
In the laboratory, levels as low as five to 15 garden symphylans per pot have been shown to reduce the growth of seedlings of crops such as snap beans, spinach, and sweet corn (Umble and Fisher, 2003a; Eltoum and Berry, 1985). The higher density of 45 garden symphylans per pot has been shown to reduce the growth of tomatoes and spinach seedlings by more than 90%.
In the field, noticeable damage often occurs if garden symphylans exceed an average of 5 to 10 per shovelful in moderately to highly susceptible crops such as broccoli, squash, spinach, and cabbage (Berry and Robinson, 1974; Umble and Fisher, 2003a).
In conventional cropping systems, two to three garden symphylans per square foot is commonly used as a treatment threshold.
Because of the considerable variability of symphylan densities within a field, sample unit counts may range from 0 to more than 100. These results are helpful in locating field hot spots. In more tolerant crops, such as potatoes, beans, and small grains, garden symphylan feeding may not lead to significant damage, even at considerably higher population densities (Umble and Fisher, 2003a).
Management and Control
Many factors influence garden symphylan population levels (Howitt and Bullock, 1955; Umble and Fisher, 2003c; Getzin and Shanks, 1964; Shanks, 1966). However, because it is difficult to accurately sample populations, information about the true effects of many factors on garden symphylans is scant at best. For management purposes it is important to make a distinction between tactics that decrease populations and tactics that reduce damage to crops but may not necessarily decrease populations. In most cases, effective garden symphylan management involves establishing a balance between these two strategies. It is important to keep in mind that in most cases, after damage is noticed, little can be done without replanting. Sampling is, therefore, important in determining the proper course of action.
It is unknown whether symphylan populations may develop from transported soil or compost. These are certainly possible sources of infestation, and it is recommended to sample soil and compost (from on- or off-site) for symphylans before applying these amendments to a field. Generally, symphylan populations are thought to be home-grown and to develop over time due to favorable soil management practices.
Tactics to Decrease Populations
Reducing populations has been the focus of many studies (Howitt and Bullock, 1955; Umble and Fisher, 2003c; Getzin and Shanks, 1964; Shanks, 1966; Howitt, 1959; Peachey et al., 2002). Though no “silver bullets” have been identified, some tactics are available. Probably no method will eradicate garden symphylans from a site, and the effect of most tactics will not last longer than one to three years.
Tillage
Tillage is probably the oldest control tactic, and it is still one of the most effective (Martin, 1948;Peachey et al., 2002). Tillage can physically crush garden symphylans, thus reducing populations. Tillage may also harm populations of key garden symphylan predators such as centipedes and predaceous mites. However, in annual cropping systems, the benefits of increased predator populations from reduced tillage have not been shown to be as effective as tillage in decreasing garden symphylan populations. When considering increased tillage, farmers need to balance the benefits and the costs, such as oxidized soil organic matter, compacted soil, increased expenses for time, fuel, and worn equipment.
In general, for the most effective control, till when the garden symphylans are in the surface soil and when soil moisture allows preparation of a fine seed bed. Since only a portion of the symphylan population is in the surface soil, tillage never provides complete control. However, surface populations are generally significantly lower for at least two to three weeks after tillage.
Garden symphylans are soil-dwelling centipede-like creatures that feed on plant roots and can cause extensive, often misdiagnosed crop damage. Describes life cycle, sustainable management options.

Damaged (above) and undamaged (below) broccoli planted at the same time in the same field.


Damaged (above) and undamaged (below) cucumbers planted at the same time in the same field.


Damaged (above) and undamaged (below) sunflowers planted at the same time in the same field.


Damaged (above) and undamaged peppers (below) planted at the same time in the same field.

Pesticides
Hundreds of compounds have been used against garden symphylans in the past 100 years, with varying efficacies (Howitt and Bullock, 1955). Fumigants and organophosphate pesticides have been the most effective, but many of these
are no longer registered. Pesticides may have the effect of both killing garden symphylans and repelling them from the surface soil. Less toxic pesticides (e.g., pyrethroids and other natural pesticides) have not been shown to provide acceptable control. Pesticides generally provide the greatest amount of control when they are broadcast and incorporated, though banded and injected applications can provide an acceptable level of control.
Crop Rotation
Although garden symphylans feed on a wide range of plants, they can also persist in bare soil by feeding on other soil organisms. Plants vary greatly in their susceptibility to garden symphylans. Crop rotation may partially explain seemingly sudden shifts in garden symphylan population levels. Populations decrease significantly in potato crops, even allowing subsequent cultivation of susceptible crops in rotation (Umble and Fisher, 2003c). Though no other crops
have been shown to be nearly as effective at reducing symphylan populations as potatoes, symphylan populations are lower after a spring oat (‘Monida’) winter cover crop than after a mustard (‘Martiginia’), barley (‘Micah’), or rye (‘Wheeler’) winter cover crop (Peachey et al., 2002). Mustard and spinach crops are very good hosts and may lead to increased populations in some cases. All these factors should be considered when developing a weed management plan.
Other Soil Amendments
The reported effects of common soil amendments such as manure, lime, fertilizers, and compost vary greatly and are often contradictory. Lime and fertilizers are generally accepted to have little effect on populations, while manure applications are generally believed to increase populations (Shanks, 1966). The effect of compost and organic amendments on garden symphylan populations has been variable, but at this point none have been shown to consistently and significantly reduce garden symphylan populations.
Tactics to Reduce Damage to Crops
Most plants can tolerate some level of garden symphylan feeding during all or part of the growing season, and two general tactics can help to grow healthy crops in symphylan-infested soil. These tactics are those aimed at reducing crop damage when garden symphylan populations are high, and those aimed at reducing the number of garden symphylans on crop roots during establishment, when plants are often most susceptible.
Tactics to Reduce Crop Damage when Garden Symphylan Populations Are High

Potatoes planted under broccoli at UC Davis Student Farm.

Potatoes planted between blocks of sweet corn in intercrop trial at UC Santa Cruz Farm and Garden. Photo by Jim Leap.
Crop Species/Variety
Susceptibility to garden symphylan feeding can vary dramatically between different plant species and varieties. In most cases tolerance to feeding seems due to increased vigor and/or root production (e.g., broccoli, corn) (Umble and Fisher, 2003a; Simigrai and Berry, 1974). In some cases garden symphylans may simply eat less of certain crops/varieties, though this has not been demonstrated experimentally. Generally, smaller-seeded crops tend to be more susceptible than larger-seeded crops (Umble and Fisher, 2003a). Commonly damaged crops include broccoli and other cole crops, spinach, beets, onions, and squash.
Beans and potatoes are rarely damaged, even under high populations. Perennial crops, such as strawberries, raspberries, hops, and bare root trees (nursery production), can also be damaged, particularly during establishment. Within a crop species, such as broccoli, some varieties are more tolerant of garden symphylans than others (Simigrai and Berry, 1974).

Perennial crops also can be damaged by symphylans are present. such as these blueberries.

Damage on hybrid poplars.
Crop Stage
Within a crop, susceptibility is often related to the developmental stage of the crop. For example, within a tomato variety, direct-seeded tomatoes are more susceptible than 4-week-old transplants, which are more susceptible than 12- week-old transplants. Using transplants or increasing transplant size to reduce
damage is not effective for all crops. Transplants of broccoli and eggplant, for example, often fail to establish under high garden symphylan populations.
Plant Density
Garden symphylans do not cross the soil surface for significant distances, as do ground beetles. However, they are quite active and surprisingly mobile for their size, moving vertically and horizontally through the soil profile. This is strikingly evident when, for example, seedlings transplanted into a stale seedbed with seemingly few garden symphylans have garden symphylans crawling all over their roots less than one day after planting.
The number of garden symphylans feeding on each plant in a local region (e.g., raised bed) is partially a factor of the number of plants present in that bed. In some cases, increasing plant density—which of course must be balanced with plant competition considerations—brings about improved production. Modifications of this strategy include planting an early “distraction” or “dilution” crop in a bed or adjacent to a cropping row.
A good dilution crop is a low-cost, vigorous, easy-establishing crop (e.g., sudangrass in suitable conditions) that increases the roots in the soil and effectively “dilutes” the garden symphylans enough to get the target crop established. The dilution crop is then removed as the target crop establishes.
Tactics to Reduce Access of Garden Symphylans to Crop Roots
Since garden symphylans are not able to burrow through soil, instead relying on soil pores and channels made by roots and other soil organisms, their access to roots is strongly correlated with soil structure, bulk density (“fluffiness”) of the soil, and pore connectivity. In general, the following tactics focus on temporarily reducing the number of garden symphylans in the surface soil before planting, thus allowing crops to establish while garden symphylan numbers are low.
Tillage
Along with directly killing garden symphylans, tillage breaks apart soil aggregates, modifying soil pores and pore connectivity. The effects of tillage vary with the types of implements used. In general, the more disruptive the tillage, the greater the effect it will have on garden symphylans. Plowing or discing, followed by thorough preparation of a fine seed bed using a rototiller or roterra, often reduces surface-feeding garden symphylan populations for two to three weeks. Over this period of time, pores are formed as some aggregation occurs, and earthworms and plant roots make new channels through the soil. Less intense soil disturbance, such as hand digging or shallow cultivation with a harrow or strip tiller, may have
a significantly less disruptive effect on garden symphylans.

Plants growing in the compacted soil of tire tracks in an otherwise bare, symphylans-infested field.
Compaction/Raised Beds
The protection of plant roots from garden symphylans is sometimes evident in zones where tractor tires have compacted the soil, or in areas where a rototiller or disc has formed a compacted layer or “plow pan.”
Although compaction can have some negative effects, in some soils it is possible to compact the soil beneficially using, for example, a landscaping roller, thus reducing garden symphylan movement enough to allow plants to establish. The opposite conditions often occur in raised beds that are highly amended with organic matter, where the soil is very low in bulk density and garden symphylans are able to move freely throughout the beds.
References
Berry, R. E. 1972. Garden symphylan: Reproduction and development in the laboratory. (Scutigerella immaculata). Journal of Economic Entomology. Vol. 65. p. 1628-1632.
Berry, R. E. 1973. Biology of the predaceous mite, Pergamasus quisquiliarum, on the garden symphylan, Scutigerella immaculata, in the laboratory. Ann. Entomol. Soc. Am. 66: 1354-1356.
Berry, R. E., and R. R. Robinson. 1974. Biology and control of the garden symphylan. Oregon State University. Extension Service. Extension Circular 845.
Edwards, C. A. 1957. Simple techniques for rearing Collembola, Symphyla and other small soil inhabiting arthropods, pp. 412-416. In D. K. M. Kevan [ed.], Soil Zoology. Butterworths Publications Ltd., London.
Edwards, C. A. 1958. The ecology of Symphyla: part I. populations. Entomologia Experimentalis et Applicata 1: 308-319.
Edwards, C. A. 1959a. Keys to the genera of the Symphyla. Journal of the Linnean Society XLIV: 164-169.
Edwards, C. A. 1959b. The ecology of Symphyla: part II. seasonal soil migrations. Entomologia Experimentalis et Applicata 2: 257-267.
Edwards, C. A. 1961. The ecology of Symphyla: part III. factors controlling soil distributions. Entomologia Experimentalis et Applicata 4: 239-256.
Edwards, C. A. 1990. Symphyla, pp. 891 – 910. In D. L. Dindal [ed.], Soil biology guide. Wiley, New York.
Edwards, C. A., and E. B. Dennis. 1962. The sampling and extraction of Symphyla from soil, pp. 300-304. In P. W. Murphy [ed.], Progress in Soil Zoology. Butterworths, London.
Eltoum, E. M. A., and R. E. Berry. 1985. Influence of garden symphylan (Symphyla: Scutigerellidae) root injury on physiological processes in snap beans. Environ. Entomol. 14: 408-412.
Filinger, G. A. 1928. Observations on the habits and control of the garden centipede, Scutigerella immaculata, Newport, a pest in greenhouses. J. Econ. Entomol. 2: 357-360.
Filinger, G. A. 1931. The Garden Symphylid. Ohio Agr. Exp. Sta. Bul. 486: 1-33.
Getzin, L. W., and C. H. Shanks. 1964. Infection of the garden symphylan, Scutigerella immaculata (Newport), by Entomophthora coronata (Constantin) Kevorkian and Metarrhizium anisopliae (Metchnikoff) Sorokin. J. Insect Path. 6: 542-543.
Gould, G. E., and C. A. Edwards. 1968. Damage to field corn by symphylans. Proc. Indiana Acad. Sci. 77: 214-221.
Howitt, A. J. 1959. Control of Scutigerella immaculata (Newport) in the Pacific Northwest with soil fumigants. J. Econ. Entomol. 52: 678-683.
Howitt, A. J., and R. M. Bullock. 1955. Control of the garden centipede. J. Econ. Entomol. 48: 246-250.
Illingworth, J. F. 1927. Symphylids destructive to the roots of pineapple. Pineapple News Mar-Dec: 88-91.
Koontz, F. R. 1968. Biological and ecological relationships of the fungus, Entomophthora coronata (Constantin) Kevorkian, and the garden symphylan,
Scutigerella immaculata (Newport). Ph.D. dissertation, Oregon State University, Corvallis.
Martin, C. H. 1948. Movement and seasonal populations of the garden centipede in greenhouse soil. J. Econ. Entomol. 41: 707-715.
Michelbacher, A. E. 1935. The economic status of the garden centipede, Scutigerella immaculata (Newp.) in California. J. Econ. Entomol. 28: 1015-1018.
Michelbacher, A. E. 1938. The biology of the garden centipede Scutigerella immaculata. Hilgardia 11: 55-148.
Michelbacher, A. E. 1939. Seasonal variation in the distribution of two species of Symphyla from California. J. Econ. Entomol 32: 53-57.
Michelbacher, A. E. 1949. The ecology of Symphyla. The Pan-Pacific Entomol. 25: 1-12.
Peachey, E., A. Moldenke, R. D. William, R. Berry, E. Ingham, and E. Groth. 2002. Effect of cover crops and tillage system on symphylans (Symphyla: Scutigerella immaculata) and other soil biota in agricultural soils. Appl. Soil Ecol. 21: 59-70.
Riley, H. K. 1929. The greenhouse centipede. Indiana Agr. Exp. Sta. Bul. 331: 1-14.
Scheller, U. 1986. Symphyla from the United States and Mexico. Texas Mem. Mus., Speleol. Monogr., 1: 87-125.
Sechriest, R. E. 1972. Control of the garden symphylan in Illinois cornfields. J. Econ. Entomol. 65: 599-600.
Shanks, C. H. 1966. Factors that affect reproduction of the garden symphylan, Scutigerella immaculata. J. Econ. Entomol. 59: 1403-1406.
Simigrai, M. and R.E. Berry. 1974. Resistance in broccoli to the garden symphylan. J. Econ. Entomol. 67: 371-373.
Stimmann, M. W. 1968. Effect of temperature on infection of the garden symphylan by Entomophthora coronata. J. Econ. Entomol 61: 1558-1560.
Swenson, K. G. 1965. Infection of the garden symphylan, Scutigerella immaculata, with the DD-136 nematode. J. Invert. Path. 8: 133-134.
Umble, J. R., and J. R. Fisher. 2003a. Influence of below-ground feeding by garden symphylans (Cephalostigmata: Scutigerellidae) on plant health. Environmental Entomology 32: 1251-1261.
Umble, J. R., and J. R. Fisher. 2003b. Sampling considerations for garden symphylans, Scutigerella immaculata Newport, in western Oregon. Journal of Economic Entomology 96: 969-974.
Umble, J. R., and J. R. Fisher. 2003c. Suitability of selected crops and soil for garden symphylan (Symphyla, Scutigerellidae: Scutigerella immaculata Newport) population development. Journal of Applied Soil Ecology 24: 151-163.
Waterhouse, J. S. 1969. An evaluation of a new predaceous centipede Lamyctes sp., on the garden symphylan Scutigerella immaculata. Can. Entomol. 101: 1081-1083.
Waterhouse, J. S., R. Seymour, and E. W. Rutkowski. 1969. Biological effects of starvation on the garden symphylan. J. Econ. Entomol. 62: 338-341.
Woodworth, C. W. 1905. A new centipede of economic importance. Calif. J. Tech. 6: 38-42.
Wymore, F. H. 1931. The garden centipede. California Experiment Station Bulletin 518: 1-22.
Symphylans: Soil Pest Management Options
By Jon Umble, Oregon State University; Rex Dufour, NCAT; Glenn Fisher, Oregon State University; James Fisher, USDA/ARS; Jim Leap, University of California, Santa Cruz; Mark Van Horn, University of California, Davis
©NCAT
Published February 2006
IP283
This publication is produced by the National Center for Appropriate Technology through the ATTRA Sustainable Agriculture program, under a cooperative agreement with USDA Rural Development. This publication was also made possible in part by funding from a Research and Education grant from the USDA’s Western Sustainable Agriculture Research and Education program (Western SARE). ATTRA.NCAT.ORG.
Photos by John Umble, unless otherwise noted.

