Organic Poultry Production: Providing Adequate Methionine

Photo: Alisha Staggs
By Anne Fanatico and Kevin Ellis, NCAT Agriculture Specialists
Abstract
Because synthetic amino acids are not allowed in organic livestock production, and animal slaughter by-products cannot be used in organic feed, nutrition can present challenges in organic poultry production. Providing the amino acid methionine (MET) without oversupplying protein is generally the most difficult. Feed rations that are high in plant proteins, such as soybean meal, can be used instead of synthetic MET, but high-protein diets are not healthy for poultry or the environment. Diets containing fishmeal, milk products, and nonconventional sources of protein, such as earthworms or insects, can help provide MET, but the ingredients are expensive and, in most cases, not available in organic form. It is difficult to design diets with sufficient MET and balance of other essential amino acids.
Contents
Introduction
Synthetic Methionine
Natural Methionine Supplement
Methionine Requirements of Poultry
Methionine Deficiency Problems
Methionine in Feedstuffs
Formulating Diets and Feeding Strategies
Conclusions
References
Appendices
Introduction
All commercial livestock production operations, organic or otherwise, must balance product quality with production efficiency (weight gain, feed cost and efficiency, etc.) for adequate economic returns. Organic production is based on use of natural sources of plant and animal nutrition, with a few exceptions where limited quantities of synthetic substances may be used for specific purposes. One such instance is the allowed use of the synthetic form of the amino acid methionine for poultry, which may be difficult to supply naturally.
Under the USDA National Organic Program (NOP), poultry must be raised with outdoor access, fed certified organic feed and given no animal-slaughter byproducts, antibiotics, drugs or synthetic parasiticides. Poultry are omnivorous in nature but are sometimes fed vegetarian feed in commercial production. According to Sundrum (2006), organic poultry production focuses on animal health and welfare, good environmental practices, and product quality, and less on economic measures such as reducing costs and maximizing production (weight gain, feed efficiency, etc.). However, economic returns are a concern for any commercial operation. For more information on organic poultry requirements, see ATTRA’s Organic Poultry Production for Meat and Eggs
205.603 Synthetic substances allowed for use in organic livestock production
(d)(1)DL-Methionine, DL-Methionine-hydroxy analog, and DL-Methionine-hydroxy analog calcium (CAS #’s 59-51-8, 583-91-5, 4857-44-7, and 922-50-9)- for use only in organic poultry production at the following maximum levels of synthetic methionine per ton of feed: Laying and broiler chickens-2 pounds; turkeys and all other poultry-3 pounds.
In the U.S., synthetic methionine (MET) has been the only synthetic amino acid allowed in organic livestock production, and only in poultry. When the NOP organic rule was first published in 2002, the MET allowance was to end in 2005. An extension was granted until 2008 and then again until October 2010. Finally, in 2012, a final rule was published to allow synthetic methionine to be added into feed at a level not to exceed two pounds per ton for chickens (laying hens and broilers) and three pounds for turkeys and all other poultry.
Proteins are made up of amino acids, and MET is an essential amino acid that is not synthesized in sufficient quantity by the animal and must, therefore, be supplied in the diet. Cysteine (CYS) is another amino acid related to MET metabolism, and together they are called the sulfur amino acids (see box below). Methionine can be provided as part of an intact protein or as a pure amino acid. It is the most limiting amino acid (or the most difficult one to supply) in a typical corn and soybean diet and is generally added in a pure form. The CYS requirement can be provided by MET. Most of the total sulfur amino acid requirement is met by the feedstuffs (about 75%), but the rest is normally supplemented by synthetic MET (about 25%). Synthetic MET is used in virtually all commercial poultry diets in the U.S., both conventional and organic.
Methionine and Cysteine Metabolism (Sulfur Amino Acids)
Methionine (MET) and cysteine (CYS) are collectively referred to as sulfur amino acids (SAA) and are involved in complex metabolic processes. Methionine is involved in the synthesis of body proteins and is a constituent of many body parts, including muscles, organs, and feathers. It is also involved in functions unrelated to protein synthesis, such as the synthesis of polyamines. In addition, MET is a methyl-group donor, helping to form dozens of compounds including epinephrine, choline, and DNA. After donation of a methyl group, through an irreversible process called transsulfuration, MET can form CYS, another amino acid needed for protein synthesis. (Two cysteines bonded together form cystine.) Although not technically an essential amino acid, CYS synthesis is inadequate when poultry diets are deficient in MET. The requirement for MET can be satisfied only by MET, whereas that for CYS can also be met with MET. Betaine and choline are nutrients that are involved with MET metabolism and save or spare some MET.
Synthetic Methionine
Synthetic MET is manufactured as a pure amino acid. Common forms are DL-MET and 2-hydroxy-4 (methylthio) butanoic acid (a.k.a. methionine hydroxyl analogue free acid). Raw materials for DL-Met include oil, natural gas, air, and water which are used to make propene, sulphuric acid (H2S), methanol (CH3OH), and ammonia (NH3) for manufacture of DL-MET (Binder, 2003). DL-Methionine (the “DL” refers to the racemic mixture), comes in a crystalline form and is 99% available MET. It is available from the companies Degussa and Adisseo. Methionine hydroxyl analogue comes in a liquid form and is 88% available MET. Synthetic MET is so pure that even in non-organic rations where no restrictions apply to its use, only five pounds of DL-MET are needed per U.S. ton of feed. However, in organic rations it is limited to two pounds per ton in chicken feed, and three pounds per ton in turkey and other poultry rations.
Products with zinc methionine available from Zinpro are listed by the Organic Material Review Institute (OMRI) as being permitted in organic livestock production. 4-Plex-E has 20% MET (directions for feeding poultry are 1.6 pounds per U.S. ton). Zinpro-E has 40% MET (directions for feeding poultry are 0.4 pounds per U.S. ton). Because feeding directions are intended to satisfy zinc levels, only a small amount of MET is provided.
Natural Methionine Supplement
Possible methods for developing a natural MET supplement include fermentation, extraction, or protein hydrolysis.
Many amino acids are produced commercially by bacterial fermentation. Because genetically modified organisms are not permitted in organic production, any MET-producing bacteria would need to be naturally selected. However, high levels of MET are toxic to bacteria, so the yields from fermentation are very low and not cost effective.
MET can be extracted from intact proteins or isolated from partially hydrolyzed proteins, but there are no such products on the market for livestock.
Methionine Requirements of Poultry
Poultry do not have specific requirements for crude protein levels; only amino acid levels. Amino acid requirements are usually presented as percentages of the diet. They may also be presented as a percentage of the protein requirement. The National Research Council’s (NRC) Nutrient Requirements of Poultry is commonly used in the United States. The requirements of broilers are given for starter, grower, and finisher phases, because the requirements change as the bird grows (less amino acids and more energy are required with age). Overall crude protein levels of 23, 20, and 18% are used for starter, grower, and finisher phases, respectively. The MET and CYS requirements are listed in Table 1.
| Table 1: Sulfur amino acid requirements of broilers* (fast-growing broilers raised in an environmentally controlled indoor environment) |
|||
| Starter | Grower | Finisher | |
|
% |
% |
% |
|
| Methionine |
0.50 |
0.38 |
0.32 |
| Methionine + cystine |
0.90 |
0.72 |
0.60 |
| Source: NRC, 1994 *”Broilers” are chickens that are young birds, tender enough to be prepared by fast cooking methods such as broiling. |
|||
The NRC lists the total amino acid requirement rather than digestible amino acid. Baker (1997) specifies digestible amino acid requirements for broilers. In the starter phase, they are 0.41% for MET and 0.41% for CYS.
Protein and amino acid requirements vary considerably according to the productive state of the bird, that is, the rate of growth or egg production. For example, a mature rooster is bigger than a hen; however, the laying hen has higher amino acid requirements due to egg production.
Factors affecting responses of poultry to amino acids include environmental temperature, dietary factors, immunological stress, age, species, genetics, and gender. These factors either influence feed intake or reduce the efficiency of use of an amino acid (D’Mello, 1994). For example, male meat birds need more essential amino acids and feed than females.
Amino Acid Balance
For optimal growth, poultry need feed rations with an appropriate balance of amino acids. An undersupply of a single essential amino acid will inhibit the responses to those in adequate supply. In poultry, lysine is used as the reference amino acid, and amino acid requirements can be expressed as a percent of lysine (100%). For example, methionine plus cysteine should equal 77.5% of the lysine level for a chick at zero to three weeks (Cole and Lunen, 1994). The ratio of MET to CYS should be about 60:40. Ideal proteins differ for broilers, layers, turkeys, and other types of birds.
Nutrient Balance and Feed Intake
Energy-to-protein ratios are important to feed rations. Energy is provided mainly by carbohydrates and fats in the diet. If the diet is well-balanced, the bird eats primarily to satisfy its energy requirements. However, if the diet is deficient in protein in relation to its energy content, the bird will overeat energy in an effort to obtain sufficient protein.
Dietary energy exerts its effect through variations in feed intake (Emmert, no date). As dietary energy levels increase, feed intake decreases. A high-energy diet effectively limits feed intake, which also limits protein and amino acid intake. Therefore, a high dietary concentration of amino acids is needed for high-energy diets. The NRC nutrient requirements are based on high-energy, high-protein (high amino acid level) diets.
In contrast, as energy decreases, feed intake increases, requiring a lower concentration of nutrients in the diet. If low concentrations of amino acids are used, diets should be low-energy so that feed intake will be increased.
Birds eat less when it is hot and more when it is cold (NRC, 1994). Therefore, diets usually need to be higher in amino acids during hot weather in order to make sure birds get enough.
Feeding diets deficient in essential amino acids can also increase feed intake. Cherry & Siegel (1981) fed pullets diets that were equal in energy and contained 15% crude protein and only differed in levels of MET and SAA. They found that the pullets compensated for a marginal deficiency of SAA by increasing their feed consumption, and that the SAA requirement for maximum feed conversion efficiency was greater than the requirement for egg production.
According to Larbier and Leclercq (1994), small birds such as leghorns are able to keep energy intake constant even with varying levels of dietary energy concentration, but heavy genotypes cannot (their feed intake is more constant).
Nutrient balance has an important impact on the carcass. In general, diets high in energy produce fat carcasses, and diets high in protein lead to lean carcasses. But again, the protein-to-energy balance is important. If a bird consumes excess energy compared to protein, a fatter bird develops (Leeson and Summers, 1991).
Methionine Requirements of Slow-Growing Meat Chickens
In the United States, maximum protein accretion is the goal both in conventional and most organic poultry production. The fast-growing Cornish Cross broiler is used in both conventional and organic production because of its high growth capacity when raised on a high supply of amino acids. In contrast, slow-growing meat chickens are used in the European Union organic program, the French Label Rouge program, and American producers of heritage breeds. In contrast to harvest of birds at eight weeks typical of fast-growing birds, these birds have a growing period of about 12 weeks. Small farms in the United States typically use Cornish Cross broilers. However, some are interested in slow-growing breeds such as Red Broilers or New Hampshire.
The nutrient requirements of high-yielding broilers raised in controlled indoor environments are well-known. In contrast, the nutrient requirements of lower-yielding meat chickens raised in less-controlled housing with access to the outdoors and a high level of activity are not as well-known. Peter et al. (1997) found that a protein level of 20% is adequate as a starter for slow-growing birds. After six weeks, protein content can be reduced to 17.5%.
U.S. research was conducted to determine the MET requirements of slower-growing meat chickens. Fanatico et al. (2006) raised three genotypes with different growth rates (fast, medium, and slow), using graded levels of MET, and found that, based on feed efficiency and weight gain responses, the MET and SAA requirements of the various genotypes are similar during the starter and grower phases.
Han and Baker (1991) found that slow-growing meat chickens require the amino acid lysine at the same concentration as fast-growing broilers. However, the fast-growing broilers required more than twice as much daily lysine as the slow-growing meat birds; the increased need was supplied by greater daily feed intake.
The body composition of meat chickens may come into play when considering amino acid requirements. If the protein-to-fat ratio of the bird is greater in a fast-growing chick than in a slow-growing chick, then dietary amino acid requirements may be higher for the fast-growing chick.
Laying Hens
The requirements for layers are given on the basis of feed intake. For example, 0.30% MET and 0.58% SAA are required for lightweight leghorn-type layers that consume 100 grams (0.22 pounds) of feed per day. Laying hens have lower MET requirements than meat birds. During weeks 16 to 22, layers are still growing and, at the same time, laying eggs and will require elevated levels of methionine.
Turkeys
Turkey poults have very high amino acid requirements to meet the demands of their rapid growth. It can be hard to get sufficient amino acids into poults in the starter phase because feed intake is low, and the poults need to accrete a lot of protein. This is especially difficult to do without synthetic MET. From zero to four weeks, 0.55% MET and 1.05% SAA are required. Amino acid needs of turkeys differ substantially by gender.
Methionine Deficiency Problems
The protein and amino acid concentrations presented as requirement by the NRC are to support maximum growth and production in fast-growing meat birds. Achieving maximum growth and production may not always ensure maximum economic returns, particularly when prices of protein sources are high (NRC, 1994). And maximum economic returns may not be the only goal. For some producers, product quality (flavor and nutrition) may be as significant as quantity produced.
According to Sundrum (2005), there are no effects on animal health from feeding a suboptimal diet or low-nutrient diet, but the birds may not be fully realizing their genetic potential. In fact, breeding companies that sell specialty birds usually show expected growth performance on a high-nutrient diet as well as a low-nutrient diet designed for conventional and specialty production, respectively.
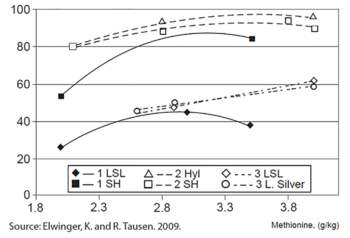
Figure 1. Feather cover (% of body size). Source: Elwinger and Tausen, 2009
Low-nutrient diets or feed restrictions are often used in the starter phase to slow the growth of fast-growing birds in order to reduce metabolic disorders and lameness. Feed restrictions have also been implemented to reduce feed spillage and better document feed efficiency. Feed density can be increased later for compensatory gain (Sundrum, 2005). A bird’s ability to adapt to variations in feed supply still exists (Sundrum, 2005). However, fast-growing, high-yielding animals are more sensitive to suboptimal feed rations than slower-growing or low-yielding animals. Stress levels may increase due to sudden changes in a feed ration, leading to depressed levels of growth.
U.S. organic poultry companies are concerned that fast-growing birds with reduced MET levels in their diet will not only perform poorly, but will also suffer impaired immune function, resulting in poor feathering, feather pecking, cannibalism, and mortality.
The antioxidant mechanisms of sulfur amino acids and their compounds are important. Normally, cells are equipped with antioxidant mechanisms to deal with free radicals. If antioxidants are out of balance, problems can occur that cause decreased animal performance. Sulfur-containing compounds such as MET and CYS are powerful antioxidants that can prevent damage in cells (Anon, 2009).
Elwinger and Tausen (2009) found that reduced MET levels reduce feather cover and egg weight, although the production of eggs was not affected. See Figure 1. They also found that feed intake increased as feather cover deteriorated, thus reducing feed efficiency.
Ambrosen and Petersen (1997) studied the impact of protein levels in feed (11% vs 19% crude protein) on cannibalism and plumage quality. The plumage improved with increased protein. Chickens supplemented with MET had better plumage quality and reduced feather pecking compared to the MET-deficient birds. However, Biedermann et al. (1993) did not show poor feathering with low protein levels. There are many factors involved in feather pecking beside the nutrient level, such as stress derived from living conditions. Feather pecking may occur even on farms with high levels of MET in the diet, for unrelated reasons.
Methionine in Feedstuffs
Methionine and cysteine are present as intact proteins in various feedstuffs. (Methionine is expressed as a percent of the feed ingredient or as a percent of the protein in the feed ingredient.) Corn is low in MET (0.17%), and soybean meal is moderate (0.64%). See Table 2 for MET and CYS contents of various feedstuffs.
As mentioned earlier, the amount of total sulfur amino acids (MET + CYS) in feedstuffs should be considered instead of only MET. If CYS is inadequate, some of the MET will be used to satisfy that requirement.
The digestibility of various feedstuffs is important when developing a feed ration. Amino acids are more digestible in some ingredients than others. Methionine in most ingredients, such as in corn and soybean meal, is highly digestible (91%), but in flaxmeal, MET digestibility is only 82%, and in sesame meal only 42%.
|
Table 2: Methionine and cysteine content of feedstuffs |
|||
|
Met |
Cys |
Notes |
|
| Soybean, full-fat, extruded |
0.48 |
0.56 |
|
| Soybean meal, expelled |
0.54 |
0.59 |
|
| Fishmeal, menhaden |
1.68 |
0.5 |
|
| Yeast, brewers dried |
0.64 |
0.43 |
|
| Casein |
2.56 |
0.4 |
ratio MET to CYS is not good; should be 60:40 |
| Milk powder, skim |
0.79 |
0.33 |
|
| Rice |
0.22 |
0.19 |
|
| Meat and bone meal >50% CP |
0.81 |
0.58 |
|
| Meat meal >50% CP |
0.72 |
0.85 |
|
| Potato protein |
1.64 |
1.06 |
|
| Black soldier larvae |
0.9 |
||
| Algae |
1.33 |
0.55 |
|
| Rapeseed, full-fat |
0.38 |
0.46 |
|
| Soybean meal, 48% CP |
0.64 |
0.7 |
|
| Sunflower meal expeller |
0.67 |
0.49 |
|
| Sunflower, full-fat |
0.38 |
0.3 |
|
| Sesame meal |
1.06 |
0.6 |
|
| Safflower meal |
0.38 |
0.41 |
|
| Flax meal |
0.35 |
0.42 |
|
| Dried distiller grains soluble, corn |
0.51 |
0.48 |
|
| Alfalfa meal |
0.21 |
0.16 |
|
| Grass |
0.27 |
0.16 |
|
| Corn gluten meal, 60% CP |
1.46 |
1.06 |
|
| Corn |
0.17 |
0.18 |
|
| Wheat |
0.19 |
0.27 |
|
| Field peas |
0.19 |
0.31 |
|
| Whey powder |
0.17 |
0.24 |
|
| Sources: AminoDat Degussa Amino Acid database; Kratzer and Vorha, 1996 | |||
Small- vs Large-scale Organic Poultry Production
Profit margins are usually thin in poultry production, including organic production. Therefore, feed efficiency and breast yield are important measurements for companies to determine profitability. These measurements may not be as important to small producers who may have wider profit margins or who consider indirect benefits as well as direct profitability of poultry enterprises to the entire farming business. High protein diets may be too expensive for some small-scale producers. Small-scale farms generally use extensive free-range systems in which grassy areas provide additional nutrients, including live protein (insects, worms, etc.) and high-quality forages during warm months.
Plant Protein
If only plant protein is used in feed rations, more protein is required than when animal proteins are also used. However, high-protein diets are not good for the birds or the environment. High-protein diets usually lead to excess nitrogen in manure and can be hard on the bird’s digestive system. Various types of plant protein are discussed below.
- Oilseed meals, such as soybean meal, are common poultry feeds after the oil has been extracted for the vegetable oil market, leaving a high-protein meal. However, the extraction process uses chemical solvents, and the remaining meal is not permitted in organic production. Organic soybeans are produced in full-fat (roasted or extruded) or meal (expelled) form.
- Many legumes and oil seeds such as field beans, field peas, lentils, etc. have antinutritional factors (ANF), including tannins and lectins. Some ANF can be removed by processing or heat-treatment. For example, soybeans have a trypsin inhibitor and must be heat-treated to destroy it.
- Sesame meal has a high MET content (1.06%); however, the MET is not well-digested and is also low in lysine.
- Sunflower meal has a MET content similar to soybean meal. Chickens cannot remove the hull of whole sunflower seeds. A SARE producer project looked at providing MET through a combination of dehulled sunflowers, fishmeal, and crabmeal, in conjunction with small-scale methods of dehulling sunflowers, and found the approach questionable (FNE05- 54).
- Canola (a cultivar of rapeseed) meal is lower in MET than soybean meal.
- Flaxseeds have a MET content of 0.62%. Although a good source of nutrients, this ingredient should be limited to no more than 30% in order to avoid a fishy flavor in the meat or eggs.
- While corn is relatively low in MET, corn gluten meal is high (1.46% MET). Unfortunately, there is none in organic form in the United States.
- High-methionine corn has been naturally selected by the Michael Fields Agricultural Institute. Jacob et al. (2008) fed the variety 3 floury-2 MF hybrid, which averages 0.32% MET, compared to 0.18% in conventional corn. They found that synthetic MET was not needed when using high-MET corn for pullets. However, high-MET corn is also high in protein; therefore, the overall high protein content of the diet is still a problem. In addition, the high moisture and low yield of this corn variety make it economically unattractive to corn growers, and therefore relatively commercially unavailable.
- Potato protein is high in MET (1.64%), and it is a conventional by-product that is currently used in Europe, where a small percentage of feed ingredients do not have to be organic in organic livestock production. There is very little if any organic potato protein in the United States.
Animal Protein
Animal protein is high quality and a good source of MET. In a pasture setting, poultry consume many sources of animal protein, including insects and worms. In the past, animal slaughter by-products (i.e., meat and bone meal) were important ingredients in conventional poultry diets, but they are banned in organic livestock feed production. However, other animal products can be used, such as fishmeal and milk products, and these are discussed below. Insects and worms can provide high-quality protein when available on pasture.
- Fishmeal is a good source of MET (1.68%) in organic livestock production. However, there is little fishmeal available without the synthetic preservative ethoxyquine, which is not allowed in organic production. Natural substances such as tocopherol can be used to prevent rancidity. Fishmeal can be used only in small amounts because it taints the flavor of meat and eggs. Eggs or chicken marketed as “Vegetarian Fed” will not be able to use fishmeal as a source of MET.
- Dairy by-products can be high in MET and highly digestible. Because liquid milk products are not concentrated due to the presence of water, powdered or concentrated products are particularly useful. Casein is the solid residue that remains after the acid or rennet coagulation of milk. It has a very high level of MET (2.56 %) and is very high in crude protein (80%). Organic casein is not available commercially for livestock feed. Whey powder, a by-product of cheese-making after most of the protein and fat are removed, is not particularly high in MET unless the protein is concentrated.
- Dried brewer’s yeast has a moderate level of MET (0.64%).
Additional Proteins
Outdoor access is required in organic poultry production but pasture is not. Many operations provide small areas (organic regulations do not specify stocking density or area size for outdoor areas); therefore, birds may not have significant access to grassy areas. Pastured poultry producers, however, usually provide extensive outdoor access by way of small portable houses moved regularly to fresh pasture. Forage is a source of MET. Although the MET level of forage is generally low to moderate, foraging should be encouraged. Birds can also obtain high-quality protein from insects and worms on pasture.
Moritz et al. (2005) found that in the summer, forage (tall fescue, orchardgrass, red clover, and white clover) had higher MET levels than in the fall (0.31% vs. 0.17%, respectively). They compared broiler performance in diets with/without synthetic methionine and with/without feed restriction. They concluded that the ability of forage to meet the MET requirement depends on environmental conditions and subsequent feed intake. Horsted et al. (2006) found that chicory was an especially attractive forage to hens and had a moderate MET content (0.40%). The quality of forages needs to be maintained for good MET levels. In most parts of the country, forage growth slows or stops in the winter. Moritz found that the digestibility of forage varies over the seasons as well. See the ATTRA publication Pastured Poultry Nutrition and Forages for more information regarding the nutritional quality of pasture in poultry diets.
Earthworms and insects are high-quality proteins, similar to fishmeal, and high in MET. Worms and insects can convert wastes such as food scraps or animal manure to high-quality protein. While there are few commercial products, worms and insects can be produced on-farm and easily processed into a meal. However, since worm and insect meal is considered an animal by-product, it cannot be fed in certified organic poultry rations.
For those who do decide to produce worms on-farm, the remains, especially worm castings, can also be very useful soil amendments. See ATTRA’s Worms for Bait or Waste Processing (Vermicomposting) publication. Additional unconventional proteins are discussed below.
- Black soldier flies lay eggs in waste, and when the larvae hatch, they consume the waste and develop into a source of high-quality protein. The larvae can “self-harvest” because they crawl upwards and will fall into collection tubes.
- Algae is high in MET (1.33%). Chlorella has potential as a feed supplement; however, production in ponds, harvesting, and drying are challenging.
Worms, algae, and aquatic plants accumulate heavy metals at concentrations greater than in the surrounding environment, and these heavy metals could transfer to meat and eggs (DEFRA, 2006). In addition, unconventional proteins may be too expensive because of the labor and processing required. Although some of the feedstuffs discussed above are high in MET, large amounts are required to meet SAA requirements, compared to the very small amounts of a pure MET supplement. For instance, one pound of DL-MET replaces 50 pounds of fishmeal.
Formulating Diets and Feeding Strategies
It is difficult to meet SAA requirements in organic poultry production without also providing excessive protein. Supplying sufficient MET to birds with plant proteins, such as soybeans or sunflower meal, may result in diets with excessive protein levels, which can be harmful to both the birds and the environment. Birds excrete the nitrogen in protein as uric acid, which is broken down into water and ammonia. Extra water is needed to excrete excess protein, making litter wetter and promoting microbial growth. High-moisture litter creates an optimal environment for pathogens and can cause breast blisters. Excess ammonia can also cause skin and respiratory problems, which increases the susceptibility of birds to other diseases, and ammonia emissions from poultry houses are a concern for air quality that can present a risk to both livestock and worker health. Metabolizing excess protein can also be detrimental to the bird, stressing the kidneys, depending on the extent of the excess (Fanatico et al., 2009). Evidence that excess protein causes stress in birds is also seen in the increased size of adrenals (Leeson and Summers, 1991). Feeding high levels of protein, particularly fishmeal, can predispose birds to necrotic enteritis (Dahiya and Drew, 2007).
In addition, plant proteins usually have antinutritional factors that require heat treatment or other processing to remove. For example, soybeans contain trypsin inhibitors and must be roasted and extruded before being fed to poultry.
Many feeds have maximum inclusion rates beyond which ingredients cannot be fed without detrimental effects on growth and/or flavor. See Leeson and Summers (1991) for more information.
There are strategies to conserve feedstuffs with high-quality protein. When diets are formulated, a higher protein content is fed earlier in the bird’s life and then steadily decreased over time. This is to make up for the low feed intake due to developing digestive systems in the early stages of growth. Quality proteins with high digestibility (generally animal proteins) should be fed during the starter phase. Plant proteins can be used later during finishing (Sundrum, 2005). Multiple-phase feeding (beyond the usual starter, grower, and finisher phases) can be useful to more closely match the diet with nutrient requirements. A broiler’s nutrient requirements change daily, rather than only three times.
In “choice feeding,” a completely formulated feed is not provided but rather the separate ingredients. For example, corn may be offered separately from a protein concentrate mixed with vitamins and minerals. Choice feeding could help match nutrients even more closely to requirements that change daily according to temperature, stage of production, and gender. In choice feeding, homegrown organic feeds can be readily used. Many grains are provided in whole forms to reduce processing costs, improve gut health and maintain nutrient content. Special care should be taken to ensure that when unprocessed grains are provided to poultry, they do not contain any antinutritional factors. Poultry also need grit in order to break down grains into digestable sizes.
Diet Formulations
Sample diet formulations for broilers, layers, and turkeys are provided in Possibilities and Limitations of Protein Supply in Organic Poultry and Pig Production (Sundrum, 2005). However, many of the ingredients are nonorganic, such as potato protein and corn gluten meal, and these cannot be used in the United States.
See Table 3 for starter and grower organic meat-chicken diets from Europe using organic sunflower, sesame, and rapeseed, which can be difficult to obtain in the United States. Note that the MET and MET+CYS levels are lower than those recommended by the NRC.
See Table 4 for fishmeal-based organic broiler diets designed by West Virginia University for research using both slow- and fast-growing broilers. No synthetic MET is used, and all the ingredients are available in the United States.
Trials
The Methionine Task Force and its members have conducted feeding trials to test various diets. For example, Organic Valley did trials with high-MET corn and presented a poster at the 1st IFOAM International Conference on Animals in Organic Production (see Appendix). Some trials included additional betaine in an attempt to spare or reduce the need for some MET.
The Methionine Task Force has also sponsored research to develop a natural methionine product. In addition, the Task Force commissioned an 80-page literature review of methionine by researchers at California State Polytechnic University (Burns-Whitmore, 2007).
See Nutrition and Feeding of Organic Poultry (Blair, 2008) for general information on organic feedstuffs and feeding.
Conclusions
It is likely that organic poultry will not have enough methionine in the diet without synthetic methionine, or that diets will contain excessive protein to satisfy amino acid requirements. Animal proteins (dairy products, fishmeal, or insects/worms) and nonconventional proteins such as algae have an important role to play, but a natural methionine supplement would greatly reduce the problem. When formulating a ration for poultry, special care should be taken to satisfy not only the methionine requirement but to provide a correct balance of the amino acids required on a daily basis.
References
Ambrosen, T., and V.E. Petersen. 1997. The influence of protein level in the diets on cannibalism and quality of plumage of layers. Poultry Science. Vol. 76, No. 4. p.559-563.
Anon. 2009. Amino acids focus at Adisseo seminar. World Poultry. Sept. 2.
Baker, D.H. 1997. Ideal amino acid profiles for swine and poultry and their application in feed formulation. BioKyowa Technical Review. No. 9. p. 1-24.
Binder, M. 2003. Life Cycle Analysis of DL-methionine in broiler meat production. Animo News. June.
Biedermann, G. von, N. Schmiemann, and K. Lange. 1993. Investigations of the effects of plumage condition at different ages in laying hens. Archiv fur Geflugelkunde. Vol. 57, No. 6. p. 280-285.
Blair, R. 2008. Nutrition and Feeding of Organic Poultry. CAB International, Wallingford, Oxfordshire, U.K.
Burns-Whitmore, B. 2007. A Review of Recent Scientific Research of Methionine. California State Polytechnic University, Pomona.
Cherry, J. A. and P.B. Siegel. 1981. Compensatory increase in feed consumption in response to marginal levels of the sulfur containing amino acids. Archiv fur Geflugelkunde. Vol. 45, No. 6. p. 269-273.
Cole, D.J.A., and T.A. van Lunen. 1994. Ideal Amino Acid Patterns. Amino Acids in Farm Animal Nutrition. CAB International, Wallingford, Oxfordshire, U.K. p. 99-112.
Dahiya, J.P., and M.D. Drew. 2007. Balanced amino acid control NE: Part 2. Feedstuffs. July 9. p. 26-27, 29.
DEFRA. 2006. Organic egg production: A sustainable method for meeting the organic hen’s protein requirements. Project code OF0357. U.K.
D’Mello, J.P.F. 1994. Amino Acids in Farm Animal Nutrition. CAB International, Wallingford, Oxfordshire, U.K.
Elwinger, K., and R. Tausen. 2009. Low-methionine diets are a potential health risk in organic egg production. European Symposium on Poultry Nutrition, Edinburgh, Scotland, August 23-27, 2009.
Emmert, J. L. No date. Sulfur Amino Acid Nutrition of Broilers.
Ewing, W. Ray. 1963. Poultry Nutrition. 5th Edition (revised). The Ray Ewing Company, Pasadena, CA.
Fanatico, A.C., C.M. Owens, and J.L. Emmert. 2009. Organic Poultry Production in the U.S.: Broilers. Journal of Applied Poultry Research. Vol. 18, No. 2. p. 355-366.
Fanatico, A.C., P. B. Pillai, J. L. Emmert, E. E. Gbur, J. F. Meullenet, and C. M. Owens. 2007. Sensory attributes of slow- and fast-growing chicken genotypes raised indoors or with outdoor access. Poultry Science. Vol. 86. p. 2441-2449.
Fanatico, A.C., T. O’Connor-Dennie, C. M. Owens, and J. L. Emmert. 2007. Performance of alternative meat chickens for organic markets: impact of genotype, methionine level, and methionine source. Poultry Science. Vol. 86, Supplement 1. Abstract.
Fanatico, A.C., P. B. Pillai, T. O’Connor-Dennie, J. L. Emmert. 2006. Methionine requirements of alternative slow-growing genotypes. Poultry Science. Vol. 85, Supplement 1. Abstract.
Poultry Science. Han, Y., and D.H. Baker. 1991. Lysine requirements of fast- and slow-growing broiler chicks. Vol. 70, No. 10. p. 2108-2114.
Horsted, K., M. Hammershoj, and J.E. Hermansen. 2006. Short-term effects of productivity and egg quality in nutrient-restricted versus nonrestricted organic layers with access to different forage crops. Acta Agriculturae Scandinavica, Section A, Animal Science. Vol. 56, No. 1.p. 42-54.
Jacob, J. P., N. Levendoski, and W. Goldstein. 2008. Inclusion of high methionine corn in pullet diets. Journal of Applied Poultry Research. Vol. 17, No.4. p. 440.
Kratzer, F.H., and Pran Vorha. 1996. Use of Flaxseed as a Poultry Feedstuff. Poultry Fact Sheet No. 21. Cooperative Extension, University of California, Davis, CA.
Larbier, M., and B. Leclercq. 1994. Nutrition and Feed of Poultry. INRA, Paris, France.
Leeson, S., and J.D. Summers. 1991. Commercial Poultry Nutrition. University Books, Guelph, Ontario, Canada.
National Research Council. 1994. Nutrient Requirements of Poultry, 9th rev. ed. National Academy Press, Washington, D.C.
Moritz, J.S., A.S. Parsons, N.P. Buchanan, N.J. Baker, J. Jaczynski, O.J. Gekara, and W. B. Bryan. 2005. Synthetic methionine and feed restriction effects on performance and meat quality of organically reared broiler chickens. Journal of Applied Poultry Research. Vol. 14, No. 3.p. 521-535.
Peter, W., S. Danicke, H. Jeroch, M. Wicke, and G. von Lengerken. 1997. Influence of intensity of nutrition on selected parameters of carcass and meat quality of French Label type chickens. Archiv fur Geflugelkunde. Vol. 61, No. 3. p. 110-116.
Rack, A.L., K.G.S. Lilly, K.R. Beaman, C.K. Gehring, and J.S. Moritz. 2009. The effect of genotype, choice feeding, and season on organically reared broilers fed diets devoid of synthetic methionine. Journal of Applied Poultry Research Vol. 18, No. 1. p. 54-65.
Rodenburg, T.B., J. Van Harn, M.M. Van Krimpen, M.A.W. Ruis, I. Vermeij and H.A.M. Spoolder. 2008. Comparison of three different diets for organic broiler: effects on performance and body condition. British Poultry Science. Vol. 49. p.74-80.
Sundrum, A. 2005. Possibilities and limitation of protein supply in organic poultry and pig production. Organic Revision: Research to support revision of the EU regulation on organic agriculture.
Sundrum, A. 2006. Protein supply in organic poultry and pig production. In: Proceedings of the 1st IFOAM International Conference on Animals in Organic Production, St. Paul, Minnesota, Aug. 23-25. p. 195-199.
Appendix A
Evaluation of Organic Broiler and Layer Ration Formulations
Introduction
A series of ration formulations was developed to evaluate the viability of using alternative ingredients to replace the available methionine currently supplied by synthetic sources in poultry rations. The ingredients’ nutritional information came from several sources: Feedstuffs Reference Guide 2003-2004, National Research Council’s Nutrient Requirements of Poultry (1994), Ajinomoto Heartland LLC, and information supplied by several ingredient producers. The nutritional profiles that served as the basis for the formulations were derived using several sources as well.
The basis for feed formulation is developing the nutritional matrix that is the backbone for each ingredient. For each ingredient, a complete nutritional profile must be established for each of the nutritional constraints of the ration. The values shown in the attached chart, Available Methionine Content of Ingredients for Poultry, were developed using the previously identified sources of information. Typically, many evaluations have used total methionine levels rather than the available methionine values shown on this chart. This was done because many of the ingredients contain significant total methionine levels, but due to many factors, only a fraction of this methionine is available to poultry. Using the available methionine values for both the ingredients and the nutritional requirements results in the most valid ration formulations. It must be noted that both the nutritional content of ingredients and the nutritional requirements for ration formulations will vary among nutritionists and feed manufacturers. The values presented here are an attempt to establish acceptable averages for each bird type.
Available Methionine Content of Ingredients for Poultry
The range of available methionine values in ingredients that could possibly meet organic production standards (no animal by-products) is significant, from a low of 0.08% of the ingredient (as fed) for dried kelp meal to a high of 0.93% for Menhaden fishmeal. For the purposes of this evaluation, fishmeal will be considered an acceptable ingredient under the organic standards. The primary ingredients in current poultry rations are corn and soybean meal. Corn (0.16% available methionine) is extremely low in available methionine and soybean meal (0.61% available methionine) can be considered as moderate in its available methionine content. For these reasons, it is the standard in poultry rations that supplemental synthetic methionine is added. These synthetic sources typically are DLmethionine (99% available methionine) and HMB (Alimet, 88% available methionine). Due to the available methionine potencies of the supplements, the typical inclusion rate is three to six pounds per ton of the ration (0.15 to 0.3% of the ration). For the rations in this example, organic soybean oil was also used when necessary to allow more formulation freedom, even though many organic feed producers do not use soybean oil (the oil is usually supplied to the ration in the form of full-fat soybean meal).
Broiler Ration Formulation
The attached sheet summarizes a series of broiler rations that were formulated to meet a common nutrient profile. The nutrients of interest in this comparison are shown in the “Nutritional Comparison” section below each ration. The alternative ingredients chosen for these formulations were restricted to those with the greatest available methionine content. The “Other” portion of the diet is comprised of all other required ingredients: limestone, phosphate, salt, trace minerals, and vitamins.
Ration A represents what could be considered a current ration. Ration B is the resulting formulation after removing the DL methionine. In this case, several compromises had to be made to achieve a feasible solution. These compromises result in a ration that would be unsuitable for broiler production. The energy of Ration B had to be reduced by 100 kcals and the available methionine reduced by 0.04%. At the same time, the protein of the rations increased from 21 to 38% (a level which could be physiologically harmful to the birds) and the available lysine (the second essential amino acid after methionine) was increased by 102%. Ration C was formulated without DL methionine but with fishmeal allowed up to a limit of 2.5% of the ration. In this ration, the target available methionine level of 0.49% was achievable, but (as in Ration B) an energy level reduction was required, and the protein and available lysine were excessive. Therefore, Ration C should be considered unacceptable due to these surpluses. Rations D was formulated with fishmeal and corn gluten meal, while Ration E used fishmeal, corn gluten meal, potato protein, earthworm meal, and sesame meal. In Rations D and E, the target values were achievable for energy and available methionine, and the excesses in available lysine were at an acceptable level. The main concern with Rations D and E is the increase in protein of about 7 to 8%. Several nutritionists contacted were uncomfortable with protein levels that exceeded the requirements by more than 5%. It might be possible to reduce this protein excess if more alternative ingredients were available and used in the ration.
These formulations simulated a broiler ration. Turkey rations are more nutrient dense than broiler rations, but the amino acid balance is proportionally similar. It can be assumed that in attempting to formulate turkey rations with these same constraints and ingredients, there would be similar deficiencies and excesses, and most likely to a greater magnitude.
Layer Ration Formulation
The same ingredient restrictions were used for both the layer rations and the broiler rations. As with the broiler rations, Rations B and C are unacceptable due to deficiencies in energy and excesses in protein and available lysine. For the layer rations, Rations D and E, with the expanded use of alternative ingredients, proved to be feasible solutions, without the energy deficiencies and the protein and available lysine excesses seen in the broiler rations. This can be explained by the fact that layer rations are less nutrient dense than rations for broilers or turkeys.
Conclusions
This series of formulations demonstrates that synthetic methionine supplements cannot simply be removed from the rations and the remaining ingredients reformulated. The resulting imbalances would be detrimental to the birds’ health. It appears that the use of fishmeal alone as an alternative ingredient is not sufficient to replace the synthetic methionine. It did appear that potentially feasible rations could be developed with the use of alternative ingredients. The primary constraint of this last conclusion is that these ingredients are either non-existent or of extremely limited availability when they are forced to fully comply with the organic ingredients standards. And again it must be noted that these conclusion were reached using only two “average” rations. Other nutritionists will likely experience additional deficiencies/excesses when formulating specific rations.
Source: Methionine Alternative Task Force, 2003
Appendix B
Available Methionine Content of Ingredients for Poultry (%)
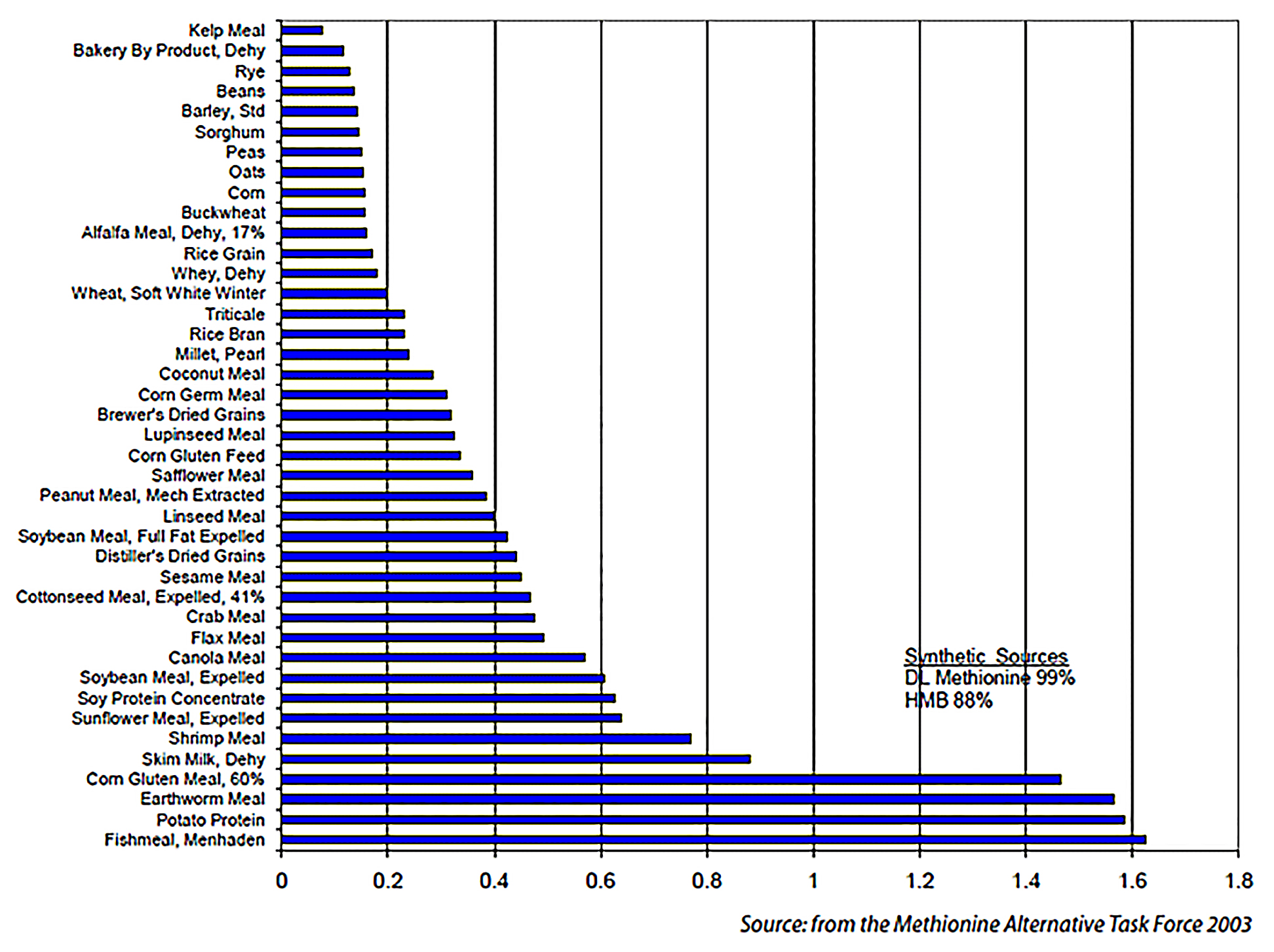
Available Methionine Content of Ingredients for Poultry (%). Source: Methionine Alternative Task Force, 2003
Appendix C
|
Broiler Ration Formulation Evaluation |
|||||
|
Ration (lbs. per ton) |
|||||
| A | B | C | D | E | |
| Corn | 1065 | 170 | 160 | 874 | 950 |
| Soybean Meal | 855 | 1720 | 1690 | 800 | 582 |
| Soybean Oil | 21 | 66 | 60 | 0 | 0 |
| DL Methionine | 4 | 0 | 0 | 0 | 0 |
| Other | 55 | 44 | 40 | 56 | 58 |
| Fishmeal | 0 | 0 | 50 | 50 | 0 |
| Corn Gluten Meal | 0 | 0 | 0 | 220 | 245 |
| Potato Protein | 0 | 0 | 0 | 0 | 0 |
| Earthworm Meal | 0 | 0 | 0 | 0 | 65 |
| Sesame Meal | 0 | 0 | 0 | 0 | 100 |
| Nutritional Comparison | |||||
| Metabolizable Energy (kcal/lb) | 1350 | 1250 | 1250 | 1350 | 1350 |
| Protein, % | 21 | 38 | 39 | 29 | 28 |
| Available Methionine, % | 0.49 | 0.45 | 0.49 | 0.49 | 0.49 |
| Excess Available Lysine, % | 0 | 102 | 108 | 25 | 11 |
| Italicized values indicate imbalances of nutritional concern | |||||
Appendix D
|
Layer Ration Formulation Evaluation |
|||||
|
Ration (lbs. per ton) |
|||||
| A | B | C | D | E | |
| Corn | 1090 | 245 | 455 | 1075 | 1310 |
| Soybean Meal | 645 | 1498 | 1245 | 465 | 40 |
| Soybean Oil | 58 | 56 | 57 | 0 | 0 |
| DL Methionine | 3 | 0 | 0 | 0 | 0 |
| Other | 204 | 201 | 193 | 215 | 210 |
| Fishmeal | 0 | 0 | 50 | 50 | 50 |
| Corn Gluten Meal | 0 | 0 | 0 | 195 | 125 |
| Potato Protein | 0 | 0 | 0 | 0 | 65 |
| Earthworm Meal | 0 | 0 | 0 | 0 | 100 |
| Sesame Meal | 0 | 0 | 0 | 0 | 100 |
| Nutritional Comparison | |||||
| Metabolizable Energy (kcal/lb) | 1325 | 1165 | 1215 | 1325 | 1325 |
| Protein, % | 18 | 34 | 31 | 22 | 20 |
| Available Methionine, % | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Excess Available Lysine, % | 0 | 107 | 88 | 0 | 0 |
| Italicized values indicate imbalances of nutritional concern | |||||
Appendix E
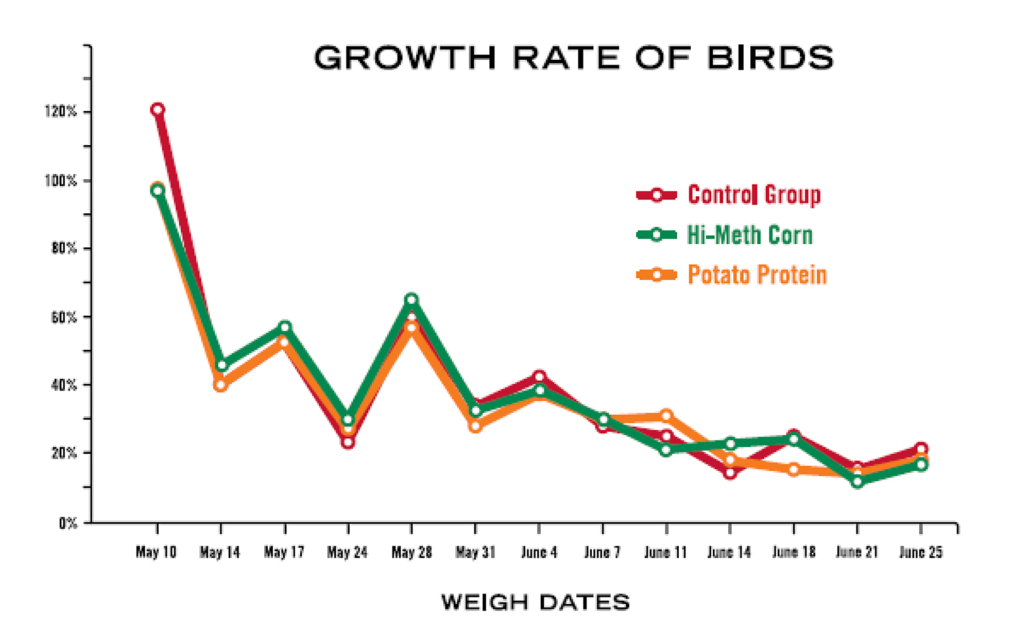
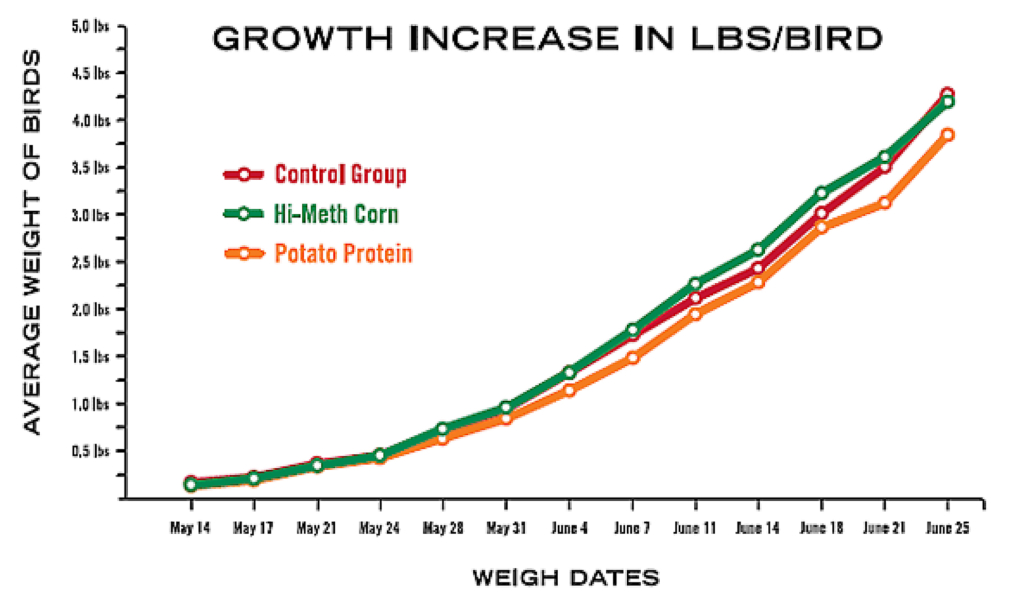
Alternatives to Synthetic Methionine Feed Trial
| Feed Rations Under Testing | |||||
|
Control Group (25 chicks) |
Hi-Meth Corn (26 chicks) |
Potato Protein (25 chicks) |
|||
| lbs | Ingredient | lbs | Ingredient | lbs | Ingredient |
| 239 | organic shell corn | 0 | organic shell corn | 275 | organic shell corn |
| 0 | organic hi methionine corn | 240 | organic hi methionine corn | 0 | organic hi methionine corn |
| 128 | corn | 140 | corn | 0 | corn |
| 13 | organic soymeal | 0 | organic soymeal | 0 | organic soymeal |
| 13 | organic flaxmeal | 12 | organic flaxmeal | 12 | organic flaxmeal |
| 50 | organic poultry builder | 50 | organic poultry builder | 50 | organic poultry builder |
| 50 | organic oats | 50 | organic oats | 50 | organic oats |
| 6 | organic barley | 5 | organic barley | 5 | organic barley |
| 1 | calcium | 3 | calcium | 3 | calcium |
| 0 | dical phos | 0 | dical phos | 105 | dical phos |
| 0 | potato starch | 0 | potato starch | 105 | potato starch |
Purpose
To find a natural alternative to synthetic DL-Methionine in organic poultry rations.
Background1
Three groups of cornish cross broiler cockerels are being given different rations. Each group is receiving the same amount of feed each day. Growing conditions are similar, all three groups are being raised on the same organic farm, in separate adjoining pens. Each batch is being weighed twice/week to measure growth patterns. Chicks arrived on May 10 as day-olds from Sunny Hatchery in Beaver Dam, WI. Changed/refilled waterers twice daily. Feed was given twice a day (splitting the total grams/day) except for the week of June 9 through 17, 1 X/day.
1Test conducted at Appley Ever After Farm, Viroqua, WI
Control Group
Good energy level, but not as high as the Hi Meth Corn group. Very good appetites—always rushed the feeder and waterer. Initially, noticed a few birds with fecal matter covering vent as chicks. (After we physically removed the matter it did not reappear.) Abscesses in wings from banding problems developed. (May have had something to do with some of the birds’ eventual total growth? ) 0% Mortality (no birds lost).
Feed Conversion Ratio: 2.77 lbs feed/1 lb gain.
Hi-Meth Corn
Highest energy level. (Initial feeding frenzies lessened when feed was increased above recommended levels.) Initially, noticed a few birds with fecal matter covering vent as chicks. (After we physically removed the matter it did not reappear.) Gained weight fastest, but Control Group caught up towards the end and surpassed the High Methionine Group at the last weighing. Although they were so close, this might have been insignificant. 3.85% Mortality—lost one bird due to neck/leg problems at the end of the trial (week 6).
Feed Conversion Ratio: 2.78 lbs feed/1 lb gain.
Potato Protein
Grew the slowest and never caught up with the Control or Hi Meth Corn groups, leading one to believe that it would take longer to grow out a broiler, or raise a layer to begin laying eggs. Feed seemed to cake up more both towards the bottom of the bags and in the feeder. Birds initially seemed to take more water than the Control or Hi Meth Corn groups, but eventually it was the same as the others. VERY calm, in comparison to the others groups. 12% Mortality: lost 3 birds due to leg/neck problems early on.
Feed Conversion Ratio: 3.28 lbs feed/1 lb gain.
Conclusion
High-Methionine Corn is a potential alternative to synthetic methionine in organic poultry rations.
Organic Poultry Production: Providing Adequate Methionine
By Anne Fanatico and Kevin Ellis, NCAT Agriculture Specialists
Published July 2016
©NCAT
IP363
Slot 363
Version 070716
This publication is produced by the National Center for Appropriate Technology through the ATTRA Sustainable Agriculture program, under a cooperative agreement with USDA Rural Development. ATTRA.NCAT.ORG.

